Towards extracellular vesicle delivery systems for tissue regeneration: material design at the molecular level
Abstract
The discovery and development of extracellular vesicles in tissue engineering have shown great potential for tissue regenerative therapies. However, their vesicle nature requires dosage-dependent administration and efficient interactions with recipient cells. Researchers have resorted to biomaterials for localized and sustained delivery of extracellular vesicles to the targeted cells, but not much emphasis has been paid on the design of the materials, which deeply impacts their molecular interactions with the loaded extracellular vesicles and subsequent delivery. Therefore, we present in this review a comprehensive survey of extracellular vesicle delivery systems from the viewpoint of material design at the molecular level. We start with general requirements of the materials and delve into different properties of delivery systems as a result of different designs, from material selections to processing strategies. Based on these differences, we analyzed the performance of extracellular vesicle delivery and tissue regeneration in representative studies. In light of the current missing links within the relationship of material structures, physicochemical properties and delivery performances, we provide perspectives on the interactions of materials and extracellular vesicles and the possible extension of materials. This review aims to be a strategic enlightenment for the future design of extracellular vesicle delivery systems to facilitate their translation from basic science to clinical applications.
Keywords
INTRODUCTION
Tissue regeneration is key to wound healing. Different conditions of tissue regeneration can lead to extremes from full necrosis to near-full recovery, during which regenerative therapies are highly critical, especially for severe injuries. For example, biomolecules, such as growth factors, cytokines[1] and microRNAs (miRNAs)[2], have been extensively explored. These signaling molecules direct intrinsic cells to attach, proliferate and differentiate into objective phenotypes. However, the administration of free biomolecules suffers from the shortcomings of fast degradation and consequent deactivation. Despite the use of delivery systems to ease the degradation problem, the paucity of biomolecules can lead to incomplete progression towards near-full recovery, unless complicated recombinant biomolecules are used[3]. Meanwhile, stem cell therapy by direct administration of exogenous stem cells into the injured tissue has also gained much attention. The full functions of the stem cells are expected to differentiate and replace the damaged tissue. However, the transplantation of stem cells alone cannot meet regenerative purposes due to the poor viability and diminished regenerative activity of the cells[4]. Such therapy can also induce immunogenicity of the body and is associated with possible ethical problems[5]. The discovery of extracellular vesicles (EVs) in 1967[6] and further investigations in 1981[7] revealed a new understanding of the communication mechanisms between cells. The EVs secreted by stem cells can act as their functional replicates and aid in the repair and protection of damaged tissue. These advances in understanding have generated research interest in using stem cell-derived EVs as intercellular vectors for regenerative therapy[8]. The use of EVs, therefore, takes the frontier in the development of tissue engineering, covering the regeneration of injured osteopathic[9], neural[10], craniofacial[11], and myocardial tissues[12]. Nevertheless, EVs are still essentially assemblies surrounded by cellular membranes and thus have to be endocytosed or contacted by the target cells to function. With this concern, four challenges exist in the use of EVs in regenerative therapies:
1) Successful delivery of EVs to the injured tissue and interactions with the target cells;
2) Long retention of EVs in the injured tissue and protection from fast clearance by organisms;
3) The dosage of the delivered EVs above a desired threshold;
4) Preservation of the bioactivity of the EVs before delivery.
The exploitation of advanced materials in tissue engineering for the delivery of drugs, biomolecules and stem cells enlightened the solution to the above challenges, which led to the rapid advancement and high effectiveness of EV delivery systems. This is reflected by many recent representative reviews of the investigations. Akbari et al. surveyed some successful cases of hydrogel-associated EV delivery and pointed out the critical role of the hydrogels in keeping the EVs and the cargoes within stable[13]. They also discerned the existing problems for the optimal approach of quantitative loading of EVs and the suitable selection of materials. Huang and coauthors focused on the procedures of the preparation of a diverse library of EV-laden scaffolds, and listed the challenges to optimally loading EVs and determining the release profile of EVs, especially in vivo[14]. Riau and colleagues provided detailed information on the approaches to loading EVs into materials and the duration of release from different delivery systems[15]. The dosage of delivery, application of artificial materials and smartness of the delivery systems were stated by the authors as perspectives. Holkar et al. provided an overview of EV-laden materials for the recovery of bone repair and presented a library of different delivery systems so far[16]. Tsintou and coauthors reviewed the cases of injectable hydrogels for prolonged delivery of EVs to achieve maximized regeneration of motor function after ischemic stroke[17]. The authors gave high remarks on EV delivery systems and urged for a translation of such material in the future. Recently, Murali and Holmes provided detailed lists of the latest EV delivery systems, their corresponding in vitro release behaviors and their regenerative performances on specific disease models[18]. The authors also pointed out issues to be addressed for the clinical translation of the delivery systems, including optimization of material designs for specific diseases, in-depth understanding of in vivo release performances, detecting possible sources affecting EVs, and resolving EV manufacturing and storage problems. In their final comments, the authors suggested a better understanding of mechanisms of action and exploration of possible optimization approaches for future EV delivery systems.
From the above excellent reviews, it can be seen that most reviews are based on the viewpoint of the therapeutic performance of EV delivery systems. In fact, the determinants of EV delivery systems are the physicochemical characteristics and structures of the materials, which govern the overall delivery performance. Therefore, we present in this review a comprehensive survey of EV delivery systems from the viewpoint of material design at the molecular level by focusing on the relationship between material structures, physicochemical properties and the performance of EV delivery. Here, “the material” is used to denote the state without EVs, and “the delivery system” stands for the state with EVs loaded. We proceeded from general to specific with the characteristics of different delivery systems as a result of material designs based on various selections of molecules and processing strategies. The materials are further classified into bulk materials and scaffolds based on the modes of loading and delivery of EVs. The according EV loading and delivery behaviors, including the release profiles in vitro, retention of EVs in vivo and the resulting tissue regeneration, were extracted from representative investigations and analyzed in correlation to the change of materials at the molecular level. From the discussions of these aspects, perspectives were also given in consideration to the current missing links in light of material structures, physicochemical properties and the performance of EV delivery, with possible extensions of materials for future designs of EV delivery systems.
WHAT ARE EXTRACELLULAR VESICLES?
Extracellular vesicles are essentially vesicles with lipid bilayer membrane structures but without functional nuclei, secreted by almost all types of organisms and cell types[19-25]. Although no consensus was reached for the assignment of EVs to particular biogenesis pathways or subtypes[19], physical properties such as physical sizes can be used to generally classify the EVs, with diameters < 200 nm as small EVs and > 200 nm all the way to 2000 nm as large ones[16,19]. EVs carry cargoes from their parental cells[16], including proteins, RNAs, DNA and cytokines, to direct the behaviors of target cells[26,27] with promoted stability and lower immunogenicity. The cargoes can also be loaded through various engineering technologies to achieve specific therapeutic purposes[28]. Thereby, stem cell-derived EVs are a better choice for regenerative therapy compared to the direct use of parental stem cells[29].
The preparation of EVs from cells is basically composed of three steps. The first is to allow the cells to secrete EVs. The conditions of growth of the cells and the cellular status have strong impacts on EVs[30-32]. Hence the correct status of the cells must be secured for the production of desired EVs. This step is followed by the isolation of EVs from the culture media of the cells[16]. Common approaches are primarily based on physical separation by the distinction in specific physical properties of EVs, including differential ultracentrifugation, density gradient centrifugation, size exclusion chromatography, ultrafiltration, flow field-flow fractionation and microchip-based techniques[16]. Some chemical or immunological based methods have also been applied, such as polymer-based precipitation, chemical-based precipitation, immunoprecipitation and immunoaffinity capture[16]. Identifications of morphological properties (electron microscopy), size (dynamic light scattering, nanoparticle tracking analysis or tunable resistive pulse sensing), surface protein markers, and proteomic and RNA cargoes are usually conducted after isolation to confirm the successful harvest of EVs[33,34]. The isolated EVs are finally cryo-preserved within antifreeze agents or lyophilized with trehalose for storage[33].
EVs have to interact with the recipient cells to exert functions, either by fusion and releasing the cargoes, initiation of downstream signaling cascade, or endocytosis after binding of EVs with the cells[8]. The cargoes of the EVs are then transferred to the recipient cells to construct crosstalk from the parental cells at molecular level, including immunomodulation, anti-inflammation, angiogenesis and specific direction of differentiation. This helps the levitation of harsh microenvironment and facilitates the recovery of the injured tissue. Nevertheless, the efficiency of such therapy is still low as simple systemic injections of EVs cannot guarantee the reception of EVs by the targeted cells despite the engineering of targeting function towards EVs. This is reflected by two factors. One is that the EVs fail to reach the recipient cells after systemic administration and are quickly cleared out from body[35], while the other is the insufficient duration of the supply of EVs, leading to degradation of most EVs by lysosome in a single administration[36]. A localized and prolonged supply of EVs in the injured tissue can ensure the delivery to the recipient cells at optimal effect. With the introduction of materials for EV delivery, such vesicles can be retained for a longer time and at sustained concentration for localized delivery.
ROLES AND REQUIREMENTS FOR EV DELIVERY SYSTEMS
In order to localize the delivery of EVs to the cells in the injured tissue and extend the whole process, the EV delivery systems are exploited to carry the EVs, transplanted and filled within the injury. Being an agent between the tissue and the EVs, the delivery systems form two interfaces with the EVs and the tissue, taking two roles oriented to each of the interfaces.
Towards the tissue, the material fills the gap created by the injury and provides mechanistic support for cells to attach after their reception of the EVs; it later degrades (if designed to do so) to provide space for further growth of new tissue. The requirements of the EV delivery systems for tissue are as follows:
1) Localized delivery of EVs: the material is applied within the gap of injury to ensure delivery of EVs to recipient cells within the local tissue;
2) Biocompatibility: the material, including the possible product of degradation, should be non-irritating, non-toxic, non-inflammatory and non-carcinogenic to the local tissue;
3) Mechanical support: the material serves as the initial attachment point of cells to migrate, proliferate and differentiate into new tissue;
4) Degradability: the material designed to be degradable should degrade either through hydrolysis, responsive decomposition or biodegradation to provide sufficient space for the regeneration of tissue;
5) Other detailed needs: based on the site of application of the material, including but not limited to mechanical strength (for bones), elasticity (blood vessel and muscle), optical properties (cornea), etc.
The role towards the EVs is the relay of EVs towards the recipient cells, which is initiated by loading, continued by storage and ended by the delivery of EVs [Figure 1]. The requirements of each step should be met to assure that the EVs reach the target:
Figure 1. Schematic illustration of the roles of EV delivery systems: EV loading, EV storage and EV delivery. EV: Extracellular vesicle.
1) EV loading: EVs should be retained within or immobilized upon the material at a high loading efficiency, with no or low possibility of release before transplantation;
2) EV storage: Upon being loaded, EVs should be well protected from possible damages, including physical (primarily mechanical) and chemical (primarily from the residue of chemicals used in fabrication) ones that will cause disruption of the vesicles;
3) EV delivery: The delivery should be to the recipient cells at a constant rate and match the kinetics of tissue regeneration. Too fast or too slow of delivery will result in either overflow or insufficient dose, both leading to deficiency of the therapy.
DESIGN STRATEGIES OF MATERIALS FOR EV DELIVERY SYSTEMS
General physicochemical properties of EV delivery systems
As the interface towards the tissue to regenerate, EV delivery systems vary with different choices of materials and functions. The key physicochemical characteristics of materials as the interface for cells are mainly focused on the mechanical and surface properties[37]. The mechanical strengths of the material can be classified into compression, tension, torsion and shearing moduli according to the directions of force, which are critical mechanical cues towards the proliferation and differentiation of stem cells and further the regeneration of new tissue[38]. For example, a hydrogel for brain tissue is required to be approximately
On the other side of the interface, the incorporated EVs have similar membrane structures to their parental stem cells, so the basic surface properties of the material still apply to them. Nevertheless, EVs do not possess cellular entities to actively start an attachment procedure as cells. Therefore, specific attention to the design of the material, especially for molecular sources and processing strategies, must be paid to secure the interactions between materials and EVs for both loading and delivery. As long as the designated biomedical properties are fulfilled, the selection of materials is non-specific to the EVs used.
EV delivery systems from polymeric sources
Water-soluble polymer-based bulk hydrogels for EV delivery systems
Hydrogels are bulk materials formed by crosslinked water-soluble polymers. Thus crosslinking is the molecular symbol of such materials. The meshes formed by the crosslinked polymer chains act as niches for the encapsulation and retention of biomolecules, as reflected by the vast studies of hydrogels as drug delivery systems[42] for different parts of body, and this is now extended to the delivery of EVs. The sizes of the meshes are mainly controlled by the density of crosslinking, including not only the number of chemical or physical crosslinking junctions in a unit volume of hydrogel but also the physical entanglement of the polymer chains, which increases with the concentration and the lengths (molecular weights) of the polymer chains. A higher crosslinking density limits the swelling ratio of the hydrogel by reducing the entropy after the swelling process[43], limiting the sizes of the meshes, increasing the number of meshes and raising the overall mechanical strengths of the hydrogel. By adjusting the crosslinking density from low to high, the mesh size can cover the order of 1 nm to 100 nm[43], and the elastic moduli range from Pa to MPa levels accordingly. Such easy customizability in both structure and mechanical properties makes hydrogels versatile to be competitive for different types of tissues, especially soft tissues such as neural and cerebral tissues[44]. The surface property of hydrogels is fundamentally hydrophilic, creating a water-abundant surface resembling that of the tissue surface and therefore improving their compatibility with EVs and coming cells. In addition, most water-soluble polymers for hydrogels can form strong hydrogen bonds with the membrane proteins of cells. In some cases, positively charged hydrogels can promote the attachment of the recipient cells of the EVs. A good example is chitosan hydrogels with protonated ammonium moieties[45-47].
Most hydrogels exploit natural water-soluble polymers as major components due to their inherent biodegradability. Reported natural water-soluble polymers include pullulan[48], chitosan[47,49], silk fibroin[50], cellulose[51], alginate[50], gelatin[52] and hyaluronic acid[53,54]. Among them, gelatin and hyaluronic acid are water-soluble extracts of decellularized extracellular matrices (ECM) and are highly compatible with EVs due to their close nature to ECM. These natural polymers are biodegradable, which is crucial for bulk hydrogels to deliver EVs, and some of them have special intrinsic properties, such as bacteriostatic and hemostatic chitosan. For water-soluble artificial polymers, past studies have mainly focused on relatively limited choices of polymers, such as polyol series [poly(ethylene glycol)(PEG)[55] and Pluronic F-127[56,57]], polyethyleneimine (PEI)[48], vinyl polymer [mostly poly(vinyl alcohol) (PVA)][57,58] and artificial peptides[59]. These polymers have specific functions based on their special structures, but are non-biodegradable except for artificial peptides. Therefore, in many cases, both artificial and natural polymers have been used to provide specific functions and overcome the shortcomings of hydrogels from a single source.
Crosslinking of the above materials is achieved through modification of the polymers or the use of special crosslinkers. Through different crosslinking structures, the hydrogel EV delivery systems can be classified and special properties be endowed. Table 1 lists the crosslinking strategies of hydrogels for EV delivery so far, including the molecular structures of the crosslinking junctions, representative materials and the corresponding properties. In addition to the single crosslinking structures shown in Table 1, some hydrogels incorporate double crosslinking network systems, especially trigger responsive ones, to achieve more controllable crosslinking and other intrinsic functions. The hydrogel developed by Li and colleagues exhibited both thermal and pH responsiveness derived from respectively Pluronic F-127 for and carboxymethyl chitosan-genipin to encapsulate human umbilical cord-mesenchymal stem cells (hUCMSCs)-derived exosomes for skin repair[73]. The thermal responsiveness leads to in situ crosslinking, while the aldehyde-amine crosslinking allows pH-responsive release of EVs to match the weakly acidic microenvironment of injuries. As for Guan and coauthors’ hydrogel, GelMA works as the main matrix and aldehyde-functionalized chondroitin mimics ECM in growth plate cartilage to supply ECM environment, promote cartilage healing with the help of bone marrow mesenchymal stem cell-derived EVs, and provide anti-inflammatory functions[74].
Crosslinking strategies, suitable selections of materials and the corresponding properties of hydrogels used as EV delivery systems
| Crosslinking strategies and structures | Representative materials | Properties | |
| (Chemical) Polymerization of methacrylamide or methacrylate | 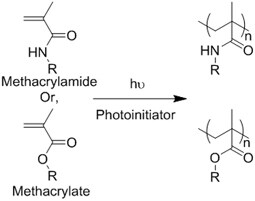 | Gelatin methacrylamide (GelMA)[52] | In situ photo-crosslinking |
| Hyaluronic acid methacrylate (HAMA)[53,60] | |||
| (Chemical) Thiol-ene reaction | Free radical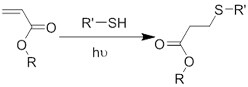 | Poly(ethylene glycol) diacrylate/thiolated alginate[61] | In situ photo-crosslinking |
Retro Michael addition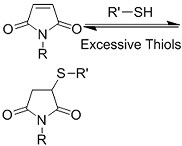 | Miktoarm PEG respectively modified with telechelic thiol/maleic acid[62] | Injectable using double-barrel syringe In situ crosslinking | |
| (Chemical) Amine-aldehyde conjugation (formation of Schiff base) | 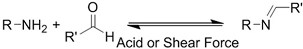 Introductions of aldehyde: Oxidation of polysaccharides: 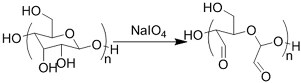 UV radiation over o-nitrobenzyl groups (fast crosslinking): 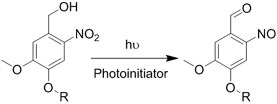 Modification with 4-formylbenzoic acid: 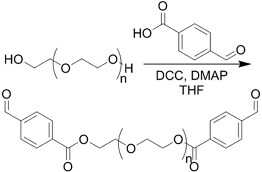 Introductions of amine: Conjugate with hydrazine: 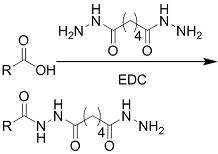 | Oxidated pullulan and PEI-modified pluronic F-127[48] Oxidated hyaluronic acid and poly-ε-L-lysine[63] Oxidated methylcellulose and PEI modified Pluronic F-127[51] o-nitrobenzyl alcohol modified hyaluronic acid and gelatin (UV responsive)[64] Chitosan and diformalbenzoic acid-modified PEG[65] Hyaluronic acid modified with adipic dihydrazide and oxidated alginate[66,67] | Injectable through shear-thinning Self-healing Tissue adhesive Acid responsive degradation (pH < 6) |
| (Chemical) Phenylboronic acid-diol conjugation | 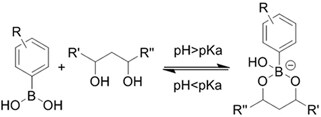 H2O2 responsiveness: 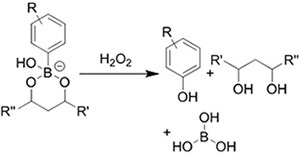 Glucose responsiveness: 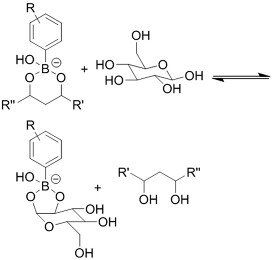 | Hyaluronic acid modified with phenylboronic acid and PVA#[68] | Injectable through shear-thinning Self-healing Acid responsive degradation H2O2 responsive degradation Glucose responsive degradation |
| (Chemical) Silver ion-thiol bonds | 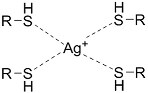 | Silver ion and thiolated miktoarm PEG[62] | Injectable through shear-thinning Self-healing In situ crosslinking |
| (Chemical) Enzyme catalysis of dopamine to form free radicals and chemical conjugation | 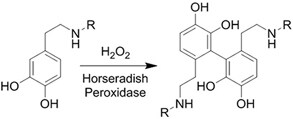 | Dopamine-modified alginate catalyzed by H2O2 and horseradish peroxidase[50] | Injectable Tissue adhesive In situ crosslinking |
| (Chemical) Enzyme catalysis of water-soluble protein to form insoluble clusters | Structure varies | Partial catalysis of fibrinogen# to fibrin by thrombin#[69] | Fast crosslinking, specific reaction |
| (Physical) Electrostatic interactions | 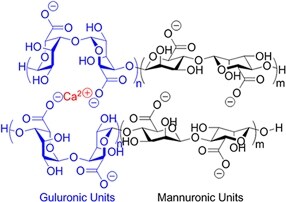 | Alginate in aqueous solution of calcium salt#[70] | Submersion in Ca2+ solution for crosslinking |
| (Physical) Temperature raising above LCST, forming hydrophobic clusters | 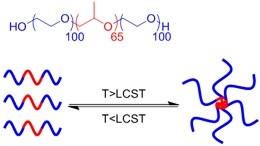 | Pluronic F-127# above 37 °C[56] | Injectable Temperature sensitive in situ crosslinking |
| (Physical) Combination of electrostatic, hydrophobic and hydrogen bonding interactions | Ionic shielding of electrostatic repulsion, enhancement of hydrophobic interaction, and redistribution of hydrogen bonding with increasing temperature | Chitosan# and β-glycerophosphate#, gelation above 37 °C[47] | Injectable Temperature sensitive in situ crosslinking |
| (Physical) Hydrogen bonds | Peptides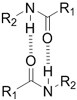 | Arginine-Glycine-Aspartate (RGD)-Biotin[59] | Crosslinking after annealing |
PVA-PEG interaction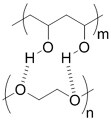 | PVA# and PEG# blend hydrogel[57] | Extrusion of PVA into PEG solution | |
Junction formation by end groups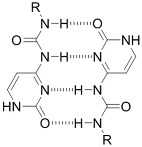 | PEG with telechelic ureidopyrimidinone[55] | Injectable pH-responsive in situ crosslinking (basic to neutral) | |
| (Physical) Host-guest interactions | 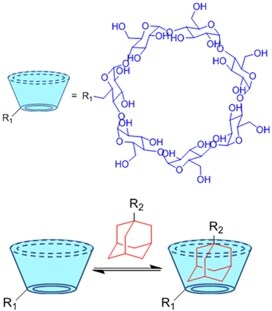 | Adamantane- and β-cyclodextrin-modified hyaluronic acid[71,72] | Injectable through shear-thinning |
With such a wide selection of polymers and crosslinking strategies, one can easily acquire hydrogels with desired properties. Such versatility makes hydrogel materials suitable for different injuries and desired regenerative therapies.
Insoluble polymer-based scaffolds for EV delivery systems
Polymeric scaffolds, in both 2D (membranes) and 3D (scaffolds) forms, serve as microporous matrices for cells to attach, proliferate, differentiate, and finally lead to the regeneration of tissue at the implanting area. The incorporation of EVs into the scaffolds can enhance the regenerative function. However, unlike hydrogels, the polymers for scaffolds are water-insoluble. Therefore, unless emulsion techniques are used (see “ADDITIONAL FUNCTIONS OF EV DELIVERY SYSTEMS”)[75], it is improper for normal-sized scaffolds to encapsulate EVs within, but provide surfaces for EVs to attach and thereafter deliver them to the recipient cells. The water insolubility and high temperature of glass transition (Tg) of the scaffold polymers lead to high elastic moduli easily reaching MPa level for both artificial polymeric scaffolds[76] and natural polymer-derived scaffolds[77], making them the choice of tissue engineering in parts requiring high tensile/compression strengths, such as bones[78], bladders[79] and dermis[80].
The major difference between hydrogels and scaffolds is the delicate porous structure. Porosity defines the overall available space within the scaffolds. Higher porosity provides more surface area for the loading of EVs. The average pore size is another indicator of the accessibility of the scaffolds. Depending on the source of the material, different porosity-introducing procedures are used. The representative materials and the corresponding processing methods are listed in Table 2. Both the porosity and the pore size can be adjusted by the bottom-up fabrication of the material (through 3D printing) or the sizes of the media to induce porosity (through sacrificial templates). The surface wettability of scaffolds is more crucial than that of hydrogels since their interactions with EVs are mainly at the surface of the material. Due to the explicit hydrophobic functional groups, polymers for the fabrication of scaffolds are mildly hydrophobic on the surface (often with static water contact angles of 60-90 °), which is slightly above the optimal range of surface tension for the adsorption of EVs (with static water contact angles approximately 40-70 ° by referring to the attachment of cells)[103]. Thus, different surface wettability is tuned depending on the type of EV to reduce excessive hydrophobicity. Generally, the adjustment of surface wettability is through coating hydrophilic materials, especially positively charged polymers, to change the explicit functional groups and thereby promote the adsorption of cells and EVs (for details, see “IMPACT OF DIFFERENT DESIGNS ON THE LOADING AND DELIVERY OF EVs”).
Representative water-insoluble polymeric materials used for EV delivery and typical approaches of porosity introduction
| Chemical nature | Materials and structures (static water contact angle*) | Approaches of porosity introduction |
| (Natural) Decellularized ECM | Bovine bone[81] (N/A) | Decellularization, followed by decalcification (bones), grinding, crosslinking and lyophilization |
| Porcine cartilage[82] (N/A) | ||
| Dermis of different animals[83] (N/A) | ||
| (Natural) Protein | Collagen#[84,85] (~92 °)[86] | Electrospinning (in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) or acetic acids) Sacrificial templates (cryogel) |
| Silk Fibroin#[87] (~74 °)[88] | ||
| (Natural) Polysaccharide | Bacterial cellulose (membrane form only)[77] (~33 °)[89] | Biofabrication by Komagataeibacter xylinus |
| (Natural) Mixture of polysaccharide and protein | Chitosan/silk fibroin[90,91] (N/A) | Sacrificial templates (cryogel) |
| (Artificial) Polyester | Polycaprolactone (PCL)#[92,93] (~75 °)[94]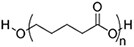 Poly(L-lactide) (PLLA)#[95] (~80 °)[96] 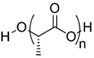 Poly(lactic-co-glycolic acid) (PLGA)#[97] (57-78 °, from 5:5 to 9:1 LA: GA)[98] 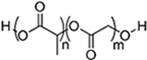 | 3D printing Sacrificial templates (sugar dissolution or cryogel) Electrospinning (in chloroform and dichloromethane, with polarity adjusted by N,N-dimethylformamide or methanol) |
| (Artificial) Polyurethane | Diol: ascorbic acid, PCL diol; Diisocyanide: hexamethylene diisocyanide[99,100] (N/A) 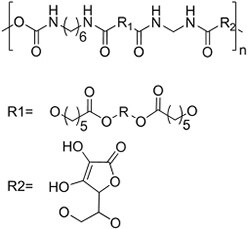 | Sacrificial templates (cryogel) |
| (Artificial) Others | Polyetheretherketone#[101] (~85 °)[102]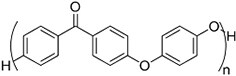 | Strong acid corrosion |
The versatility of scaffolds, unlike the wide selection of materials and crosslinking mechanisms for hydrogel, lies in the different porous structures. Changes in pore sizes, pore shapes, and connectivity of the pores, may lead to various EV loading situations and subsequently, different cell attachment, migration and communication. With fine control over the porosity-introducing, different porous structures can be further investigated for optimized regenerative results.
Hydrogel scaffolds for EV delivery systems
Hydrogels are materials encapsulating EVs, serving as bulk materials to fill the space of injuries. They have adjustable crosslinking density to precisely control the mechanical properties in resemblance to tissues, but have low surface areas for the attachment of cells for regeneration. On the other hand, scaffolds have delicate porous structures providing a large surface area for the attachment of EVs and the attachment, migration, proliferation and differentiation of cells, but the high mechanical strength limits their area of application. The combination of hydrogel and scaffold structure into hydrogel scaffolds can merge the advantages while overcoming the shortcomings.
Based on the definition of scaffolds, shear-thinning hydrogels, which self-heal after a certain period of time, are not suitable for the fabrication of hydrogel scaffolds. In situ photocrosslinkable hydrogels such as GelMA[104] and alginate methacrylate (AlgMA)[105] are the most popular choices. Similar to the fabrication of scaffolds, porosity can also be introduced through sacrificial templates or fine structure building (e.g., 3D printing). Due to the aqueous state of the polymer, ice crystals are the main choice for the templates. The overall procedure follows the route of solution freezing, photocuring and lyophilization[91]. With the swelling property, lyophilized hydrogel scaffolds exhibit a water-absorbing function when submerged in EV suspensions, providing the potential to suck EVs into the pores distributed on the surface. At the same time, pores become smaller following swelling so that the absorbed EVs are enclosed in the pores. Such behavior gives them the name of “hydrogel sponges” for the loading of EVs through absorption[91]. Shi et al. developed a chitosan (low degree of deacetylation)/silk hydrogel sponge to load EVs from gingival mesenchymal stem cells, with silk fibroin providing mechanical support, and chitosan performing the swelling function and providing cationic charges[91]. This hydrogel scaffold overcame the excessive swelling of sole chitosan and the brittleness of sole fibroin scaffolds. Similarly, a triple-layer hydrogel scaffold composed of bladder acellular matrix, partially oxidated alginate-grafted gelatin hydrogel (crosslinked by aldehyde-amine and calcium ions) and knitted silk mesh was designed by Xiao and colleagues to provide sufficient waterproofness and stretching resistance[106]. Such a structure is similar to “steel and concrete”, providing strong mechanical properties in resemblance to those of bladder.
The bottom-up 3D printing of polymer solution with simultaneous printing and crosslinking can build well-defined hydrogel scaffolds. Due to the overall non-damaging process, the EVs can be loaded either after the fabrication of scaffolds as in ordinary fabrication procedure, or can be directly mixed in the polymer solution during the preparation of bulk hydrogels, which is called “bioink”. Chen and colleagues developed a 3D-printable bioink composed of GelMA, mesenchymal stem cell-derived EVs and porcine cartilage ECM powder[104]. The resulting hydrogel scaffolds, therefore, acquired radially oriented 3D architectures as designed, which facilitated the cell ingrowth for the regeneration of cartilage with the help of mesenchymal stem cell (MSC) derived EVs. Materials of other sources, such as alginate-Ca2+ hydrogels, which do not possess in situ photo-crosslinking functions but require the addition of specific crosslinking molecules, can undergo monolayer printing-Ca2+ solution crosslinking cycles to achieve a multi-layered scaffold, as demonstrated by Sun et al. in their macrophage EV-laden alginate scaffolds for osteogenesis and angiogenesis[107].
The combination of hydrogels and scaffolds brings great diversity to the design and fabrication of EV delivery systems. This endows future EV delivery systems with high tailorability for any injury at any location.
EV delivery systems from inorganic sources
Unlike polymeric materials, inorganic materials are essential minerals with high elastic moduli. For example, hydroxyapatite, with a formula of Ca10(PO4)6(OH)2, is the major inorganic component of bones, whose elastic modulus can reach ~150 GPa in the form of single crystals under nanoindentation[108]. In consideration of the type of tissue with high stiffness and with a high abundance of mineral contents, inorganic material EV delivery systems can and have only been applied for bone regeneration. The commonly utilized inorganic materials include β-tricalcium phosphate [β-Ca3(PO4)2, β-TCP] ceramics[109], titanium oxide[110], titanium alloy[111], bioactive glass (45S5)[112], hydroxyapatite[113], and calcium sulfate[113]. These materials have been used as bone substitutes to exert osteoinductive and osteoconductive functions in tissue engineering for years, especially hydroxyapatite, the major inorganic component of bones. Some of the above materials have to be specifically processed from the raw materials to achieve the desired compounds or structure.
Inorganic materials can be used as bulk materials for the encapsulation of EVs and scaffolds for the attachment of EVs. With their low solubility, a slurry of salts and EVs can set into solid bulk forms after slight evaporation of excessive water. Qayoom and colleagues reported such a bulk salt EV delivery system fabricated by the setting of nanohydroxyapatite, calcium sulfate, human bone morphogenetic protein-2, zoledronate and bone marrow mesenchymal cell-derived EVs[113]. The slow dissolution of calcium sulfate (~0.26 g/100 g water at 25 °C for CaSO4·2H2O, decrease with temperature[114]) allows slow creation of pores and release of encapsulated EVs while providing space for the attachment and infiltration of cells. However, compared to the mode of bulk materials, more popularity lies in the mode of scaffolds in the exploitation of inorganic materials. The porosity of the scaffolds can also be introduced, again, using sacrificial templates and 3D printing. Unlike polymeric scaffolds with ice or sugar as sacrificial templates, inorganic scaffolds use polymer beads since a high-temperature sintering process is required to merge the minerals together and mold the shape. The bioglass scaffolds developed by Liu and colleagues used different polymeric templates to introduce hierarchical pores: micelle-forming Pluronic F-127 to form mesopores (7.7 nm), microspheres (0.5-2 μm) to form micropores and polyurethane sponges to form macropores (200-500 μm)[115]. All materials were mixed with mineral slurry to set and sintered to merge and burn off the polymeric templates to achieve hierarchically porous structures for the attachment of mesenchymal stem cell-derived exosomes. A similar process has been applied to commercially available porous β-TCP scaffolds[109,116]. 3D printing can combine the steps of sintering and structure-building, as demonstrated by the Ti6Al4V alloy and lithium chloride incorporated bioglass scaffolds prepared by Zhai et al.[111] and Liu et al.[112] for the attachment of EVs through incubation. For pure metals with even higher melting points, which cannot be processed by sintering or 3D printing, an even stronger method is needed. Titanium must be processed by electrochemical anodization to fabricate nanotube topography on the surface as a 2D scaffold for further attachment of EVs and bone regeneration[110]. Again, most inorganic scaffolds are treated with coatings to change the surface chemistry at the molecular level and enhance the interaction with EVs.
Compared to polymeric materials, not many inorganic materials have been tested. The primary difficulties are the relatively harsh processing procedures and the narrow window of selection. It is expected that more inorganic materials with unique properties, such as 2D materials, can be explored in the future.
EV delivery systems complexed of polymeric and inorganic materials
Most inorganic scaffolds have to be fabricated through sintering to achieve a certain volume for implantation, which requires well-controlled high temperatures. Otherwise, the raw materials are in powder form. In addition, the high stiffness of the material may not have a strong affinity for cells to attach and proliferate. Under such circumstances, the combination of polymeric matrix and inorganic material powder into complex material is favored to incorporate the advantages of both types of materials, in a way to take advantage of the maneuverability, degradability and affinity of polymeric hydrogels or scaffolds and the easy absorption of powder formed minerals by the bones. Such an approach is called “mineral-doping”. Chen et al. doped hydroxyapatite in thiolated hyaluronic acid and thiolated heparin hydrogel crosslinked by “ene” modified PEG[117]. This hydrogel was embedded with miRNA-375-carrying EVs from bone marrow mesenchymal stem cells to treat calvarial defects. Hydroxyapatite was also encapsulated within the hydrogel so that the injured bones could take advantage of the osteoinductive function for regeneration. For non-water-soluble scaffolds, Gandolfi and colleagues mixed finely milled dicalcium phosphate dihydrate and calcium silicate into PLA solution[95]. Through a phase separation technique, which involves the freezing of a polymer solution and the use of a miscible solvent with an even lower melting point to remove the frozen solvent[118], a mineral-doped PLA porous scaffold was obtained, with pore diameters ranging from 10 to 200 μm. The minerals, in the form of fine powder, are easier for osteoinduction. This scaffold was later surface-attached with EVs from mesenchymal stem cells to achieve full osteogenic function for bone regeneration. With the development of both polymeric and inorganic materials, more combinations and more functions can be expected for complex EV delivery systems.
IMPACT OF DIFFERENT DESIGNS ON THE LOADING AND DELIVERY OF EVs
For bulk materials: EV-encapsulation and release
For bulk hydrogels (including hydrogel scaffolds fabricated by the 3D printing of EV-encapsulated bioink[104]) or bulk inorganic materials, the network of materials serves as cages to retain the EVs. The caging process has to be completed before the formation of the meshes, meaning the EVs are mixed directly with the raw material to form a mixture, followed by the curing of hydrogels or the setting of inorganic mineral bulks to establish the meshes. This has been demonstrated by the procedures of preparation in many bulk EV delivery systems[52,53,61,105].
The major mode for EVs to leave bulk materials is diffusion, which is controlled by the degradation of the bulk material. Diffusion is driven by the concentration (or the chemical potential) of EVs. The initial concentration of EVs, often in the range of 109 to 1012 particles/mL, is the highest, standing for maximum potential for EVs to diffuse out of the material. This is followed by a sustained release close to a constant rate (zeroth-order), but as time passes, the concentration lowers, so that the rate of diffusion is gradually reduced and ultimately tends to zero.
In addition, the existence of meshes prolongs the diffusion of EVs for bulk materials. The average size of the meshes is generally considered smaller than the diameter of the encapsulated EVs, while a multi-layered mesh structure is also present to increase the number of meshes for EVs to diffuse through. These two structural parameters are the factors in retaining EVs. The enlargement of meshes and the decrease in mesh numbers mark the lifting of the restrictions and the start of the release of EV. Such a change in the mesh is mainly caused by the passive degradation of the material. For water-soluble polymers, degradation occurs through biodegradation by enzymes secreted by the coming cells. Due to the smaller size of cells compared to the meshes, the biodegradation of hydrogel starts from the surface of the hydrogel and then moves inward, which is an accelerating procedure with more availability of enzymes from the increasing number of attached cells and greater infiltration of enzymes into the looser and decomposed hydrogel[119], until the final remnants of hydrogels with little surface available for enzymatic reaction [Figure 2A]. For inorganic bulks, dissolution (inorganic salts) of the network is the major process limiting the diffusion of EVs [Figure 2B]. However, to the best of our knowledge, there is no reported comparison between the release of EVs versus the dissolution of salt[113]. It can be speculated that the process takes a similar curve as the biodegradation of the hydrogel. As water gradually breaks the intermolecular or interionic bonds, water molecules infiltrate more easily through the new defects from the dissolved parts until full dissolution of the salt.
Figure 2. Schematic illustration of the mode of action of bulk EV delivery systems through EV-encapsulation and release by degradation: (A) Enzymatic degradation and responsive release by hydrogels and (B) release by dissolution of inorganic bulk materials. EVs: Extracellular vesicles.
The relative relationship between the rates of EV diffusion and hydrogel degradation determines the overall release profile in vitro, which can be measured by quantification of specific antibodies[52], particle number[53] and the intensity of fluorescence labels[61] of the released EVs after specific lengths of time. At the starting stage (0-~30% accumulative release of EVs), the high potential of EVs may cause a burst release of EVs, as observed in some reported delivery systems[74,120]. As the delivery proceeds into the middle stage (~30%-~80% cumulative release of EVs), the reduced potential of EVs and the accelerated degradation cancel out, reaching a close-to-constant release of EVs, which is the key stage of bulk material delivery systems. This stage can cover three[119] to more than 30 days[62,121], depending on the material structures and the need for recovery of the injury. A well-tuned hydrogel can even fully match the release profile of EVs with the degradation profile, as illustrated by almost identical curves of material degradation and EV release in Zhang and colleagues’ HAMA hydrogel scaffold[53]. In the final stage, the chemical potential of EVs is too low to overcome the obstacle of diffusion from the remaining hydrogel, leading to non-100% release until the full degradation of the hydrogel.
At the macroscopic level of material, a high crosslinking density can both reduce the mesh size and add the number of meshes in the material, and at the same time slow down the degradation rate. This can effectively extend the retainment of EVs, as demonstrated by the faster release (~90% over 2 h) from 2% AlgMA hydrogel than that (~40% over 2 h) of 4% AlgMA hydrogel in the study by Huang and coauthors
Figure 3. In vitro EV release profiles of (A) Hydrogels without responsiveness of different crosslinking densities; (B) acid-responsive hydrogels crosslinked by amine-aldehyde conjugation; (C) enzyme-responsive hydrogels. Figure 3A adapted with permission[105], Copyright 2021, Elsevier; 3B adapted with permission under terms of the CC-BY NC license[63], Copyright 2019, the authors, published by Ivyspring International; 3C adapted with permission[122], Copyright 2019, Royal Society of Chemistry. EV: Extracellular vesicle.
In addition to the crosslinking structures and the distribution of EVs in the material, other parameters can also affect the diffusion of EVs from hydrogels. The change of stiffness of physically crosslinked hydrogel (i.e., stress-relaxing hydrogels) is one of such kinds. In their study on the impact of stiffness of hydrogel on the release of EVs, Lenzini and colleagues adjusted the Ca2+ concentration to achieve soft (shear modulus ~500 Pa) and stiff (shear modulus ~3 kPa) alginate hydrogel without a dramatic change in mesh size[70]. It was found that EVs could still diffuse through a stiff Ca2+-crosslinked alginate hydrogel compared to softer or non-stress-relaxing hydrogels. This indicated the possible diffusion by the deformity of EVs under the confinement of stiff stress-relaxing hydrogels. Such a fact also suggests that flexible polymer chains in hydrogel may also affect the diffusion of EVs. A hydrogel of polymers with low Tg, such as polyethyleneglycol (Tg around -63 °C[125]), is supposedly softer compared to hydrogels of polymers with high Tg, such as hyaluronic acid, given the same crosslinking density. However, no such study has been made to the best of our knowledge.
The tracking of in vivo release profiles is a lot more complicated compared to in vitro observations, which makes such information highly limited. The major approach to observing the traces of EVs in vivo is through qualitative or semiquantitative fluorescence signals of labeled EVs. Han et al. tracked the PKH-26 red signal of hUCMSC-EVs released by the enzyme-responsive hydrogels mentioned above in the border region of myocardial infarction[122]. The retention of EVs matched the in vitro release profile with discernable existence at day 21 in the tissue, while no trace of EVs was found for injections with sole EVs [Figure 4A]. Such labeled EVs can also be observed by live and ex vivo bioluminescence imaging to locate their distribution after release. Mardpour and colleagues encapsulated MSC-EVs in thiol-maleimide PEG hydrogels to treat chronicle liver failure[62]. The live and ex vivo imaging clearly showed maximum retention of released EVs in liver after seven days and gradual decrease till Day 30, while no trace was found for EV-only groups at the same time spots [Figure 4B]. Similarly, Zhang et al. presented the biodistribution of Gaussia luciferase labeled MSC-EVs encapsulated in amine-aldehyde chitosan hydrogels for blood vessel regeneration of ischemic hindlimbs [Figure 4C][126]. In combination with quantitative analysis results of fluorescence intensity and component (miRNA-126), the hydrogel successfully retained the EVs within the hindlimb for over 72 h.
Figure 4. In vivo tracking of EVs released from hydrogels. (A) Fluorescence microscopy of PKH-26 red-labeled hUCMSC-EVs released by the MMP-responsive hydrogel in the border region of myocardial infarction; (B) Live and ex vivo imaging of PKH-26 red-labeled EV delivery PEG hydrogels crosslinked by thiol-maleimide conjugation in the chronic liver injury model; (C) Live imaging of Gaussia luciferase-labeled MSC-EVs encapsulated in chitosan-β-glycerophosphate hydrogel injected in ischemic hindlimb. Figure 4A adapted with permission[122], Copyright 2019, Royal Society of Chemistry; 4B adapted with permission[62], Copyright 2019, American Chemical Society; 4C adapted with permission[126], Copyright 2018, American Chemical Society. EVs: Extracellular vesicles; PEG: poly(ethylene glycol); MSC: mesenchymal stem cell.
From the cases above, it can be seen that the bulk materials have been extensively applied to achieve the successful retainment of EVs. Especially for hydrogels, their release profile can be adjusted primarily through the crosslinking strategies and the crosslinking density. However, more structural parameters, such as the intrinsic flexibility of the polymers and the homogeneity of crosslinking density, can be investigated for their impact on the release performance of hydrogels. Moreover, the different delivery performance in vivo of responsive crosslinking designs is difficult to reflect in current studies. On the other hand, limited by the rare studies of inorganic bulk material, the correlation of material structure, properties and release performance is still unclear.
For scaffold materials: two different modes
As mentioned in “DESIGN STRATEGIES OF MATERIALS FOR EV DELIVERY SYSTEMS”, most EV delivery scaffolds have EVs attached. Depending on the status of EV attachment, the delivery mechanism can be divided into two modes [Figure 5].
Figure 5. Schematic illustration of two modes of action of EV delivery scaffolds: (A) Surface adsorption and release of EVs and (B) surface immobilization of EVs and cell recruitment. EVs: Extracellular vesicles.
EV-adsorption and release
The first EV attaching mode is through adsorption to scaffolds, and accordingly, the delivery is through the desorption and release. Equation 1 depicts the reversible relationship between adsorption and desorption:

where EV · Scaffold denotes the EV attached state and EV + Scaffold means EVs are released. kdstands for the coefficient of desorption and ka for adsorption. The adsorption-desorption is a reversible process. kd is affected by multiple factors, such as the concentration difference of EVs between the scaffold and the environment and the liquid shear force within the injury, which can be treated as a constant here. ka is dependent on the interactions of EVs and scaffold surface, and also the concentration difference of EVs.
In the preparation of most EV delivery scaffolds, the incubation of scaffolds with EV suspensions is the basic method to load EVs. A higher concentration of EVs can lead to a higher ka and thus a larger loading efficiency. However, when transplanted to tissue, ka significantly decreases due to almost zero presence of EVs of the same type in injured tissue. Therefore, adsorption alone is unstable and a burst release can be expected from mineral scaffolds or non-treated polymeric scaffolds[109,127,128]. An increment of ka by enhancing the EV-surface interaction is necessary for a more controlled release profile. Thus, the adsorption and desorption of EVs to different surfaces of material are crucial to their loading and release. Although previous reports have provided semiquantitative characterizations of the coverage of EVs adsorbed on scaffolds using fluorescence microscopy[97] or electron microscopy[129] as a result of adsorption, followed by the release profile of the EVs as a result of desorption, rarely direct investigations have been made, possibly due to the difficulties in the detection of nanosized EVs with sufficient resolution of the whole process[130]. The only research, to the best of our knowledge, was conducted by Yang and colleagues[130]. They developed interferometric plasmonic microscopy for imaging and size analysis of the adsorption and desorption of exosomes to negatively charged gold, positively charged gold, PEG-modified gold and CD63 antibody-modified gold surfaces. For the different charges of the surfaces, positive charge exhibited the strongest attraction of EVs from A549 cell lines, i.e., highest ka, to adsorb to the surfaces, compared to few (ka≈ 0) on negatively charged ones [Figure 6]. On surfaces with different chemical properties, EVs bounced off PEG-modified gold surfaces, as a representative superhydrophilic material with reverse-osmotic properties, while an obvious “adsorb-stay-desorb” procedure was observed for CD63 modified surfaces. This is due to the weak but insufficient interaction, or insufficient ka, between the antibody and the EVs. The results suggest that in order to promote the loading efficiency and ensure more sustained release, a properly strong interaction and kabetween the EVs and surfaces of scaffolds are required.
Figure 6. Interferometric plasmonic microscopy detection of the number of EVs from A549 cell lines binding different surfaces: (A) Illustration of the experiment; (B) EVs binding positive and negatively charged gold surfaces. Positively charged surface shows immobilization effect while negatively charged surface shows bouncing off of EVs; (C) EVs binding PEG and antibody-coated gold Surface. PEG surface shows bouncing off of EVs while antibody-coated surface shows an “adsorb-stay-desorb” procedure. Adaptations with permission[130], Copyright 2018, National Academy of Sciences. EVs: Extracellular vesicles; PEG: poly(ethylene glycol).
EVs have phospholipid bilayer membrane structures with proteins and peptidoglycan attached to or inserted within. The phosphates groups on phospholipids, and amide groups, amine groups, hydroxy groups and carboxylic groups on the proteins and peptidoglycans are able to interact with the scaffolds by hydrogen bonds or van der Waals forces when polar carbonyl groups are present, such as PCL, PLLA and PLGA. This is the case when the surface of the materials is not modified.
One way of enhancing adsorption is to raise the density of hydrogen bonds or van der Waals forces between EVs and scaffold surfaces. Polydopamine coating is the most applied method to increase the ka. Inspired by the adsorption of mussels on solid surfaces, researchers have applied polydopamines to introduce a high number of hydrogen bond donors (hydroxy on catechol groups) and exert π-π interactions (benzene of catechol groups) between surfaces[131]. In the report on poly(lactic-co-glycolic acid) (PLGA) EV delivery scaffolds for bone regeneration, Li et al. showed that the coating of polydopamine can significantly reduce the burst release of EVs from ~60% to ~20% of the total load, and retention from three days to more than eight days[97]. Since the membrane of EVs is mainly composed of phospholipids, they are hydrogen bond acceptors as well as negatively charged under physiological conditions, and thus can be adsorbed with polydopamine coating with a higher probability of extending the release [Figure 7A][97]. Tannic acid, with a similar catechol structure as polydopamine, has also been used. Under oxidizing and basic conditions, the catechol structures of tannic acid allow it to self-polymerize and become adhesive reminiscent of polydopamine, as demonstrated by the PEEK scaffolds modified by Fan et al.[101]. With the coating of tannic acid, the surface hydrophilicity (i.e., water contact angles ranging from 60 ° to 30 ° after coating) was greatly promoted. Accordingly, an obvious reduction in burst release (50% to 10% release at Day 0 after coating) and more linear release (three days to 14 days to reach 90% release after coating) were both observed [Figure 7B]. Specific bioconjugation has also been exploited to mimic natural EV-ECM complex structures[132] by applying a coating of ECM involved polymers. Heparin has been used to anchor biomolecules such as growth factors, and was recently also applied as coating of scaffolds by Wei et al to treat hyperlipidemia[93]. A heparin coating on a polycaprolactone (PCL) scaffold for blood vessel graft was implanted on the abdominal aorta. The coating slightly slowed down the release of the vesicles on the scaffold, as shown by the in vivo fluorescence live imaging of luciferase labeled EVs [Figure 8A][93]. This is one of the few reported in vivo EV release behavior of scaffolds. Similarly, the bioconjugation effect has also been reflected by the prolonged release profiles of scaffolds made from or incorporated with decellularized ECM materials, including collagen (over 14 days release)[133] and decellularized cartilage (more than 50% compared to blank, 14 days in vitro release)[104]. Another approach for increasing hydrogen bonds is through the modification of the surfaces of scaffolds, exploiting antibodies specific to the antigens on EVs, as was shown by the CD63-A549 cell line-derived EVs in the interferometric plasmonic microscopy test by
Figure 7. The in vitro release profile of EVs from scaffolds prolonged by coatings: (A) Polydopamine-coated PLGA scaffold and (B) tannic acid-coated PEEK scaffold. Figure 7A adapted with permission[97], Copyright 2018, American Chemical Society; 7B is adapted with permission[101], Copyright 2021, the authors, published by KeAi Publishing. EVs: Extracellular vesicles; PLGA: poly(lactic-co-glycolic acid).
Figure 8. In vivo tracking of EVs delivered from scaffolds. (A) Live imaging of the retained delivery of Luciferase-labeled EVs by PCL scaffold coated with heparin at abdominal artery of mice; (B) live imaging of the immobilized EVs on PCL scaffold conjugated with PEI implanted on large skin wound of mice. Figure 8A adapted with permission[93], Copyright 2019, Elsevier; 8B adapted with permission under terms of the CC-BY NC license[92], Copyright 2021, the authors, published by American Association for the Advancement of Science. EVs: Extracellular vesicles; PCL: polycaprolactone; PEI: polyethyleneimine.
Compared to weak hydrogen bonds and van der Waals interaction, electrostatic interactions are much stronger and more effective in retaining EVs. The majority of phosphate groups on the membrane of EVs make them overall negatively charged. Some scaffolds with relatively weak cationic charge can also extend the release of EVs by increasing ka through electrostatic interactions, such as chitosan (80% in six days in vitro)[90].
EV-immobilization and cell-recruitment
The fixed mode of EV attachment is less frequently utilized compared to the release mode. It involves the full immobilization of EVs on the surfaces of scaffolds for the recruitment of targeted cells instead of releasing them. In this case, ka is much larger than kd, so the overall reaction is one way to reach the
It follows that surface modifications with specific interactions with EVs have shown to greatly increase the retainment of adsorbed EVs on scaffolds, both in vitro and in vivo. This has been the mainstream for the preparation of EV delivery systems in scaffold forms. However, a deeper understanding of the intensities of the interaction between the coatings and EVs is necessary for better control of the delivery of EVs. Meanwhile, porosity, the key feature of scaffolds, has not been mentioned in any of the above-discussed studies. This parameter affects the overall loading capacity of EVs and should receive more emphasis in further investigations.
Promotion of tissue regeneration by EV delivery systems
Despite the need for both an in-depth understanding of the interaction between the delivery system and the release of EVs, and more accurate control over stimuli-responsive release, both bulk and scaffold materials have shown good prolonged EV delivery compared to the administration of only EVs and the results are obvious in the regeneration of tissue, as reflected by the many reports of their use in treating various injuries at different sites of the body.
Concerning bulk materials to treat periodontitis, Shen and colleagues injected chitosan-β-sodium glycerophosphate hydrogel encapsulating dental pulp stem cell-derived EVs into the cavity of periodontitis[47]. Compared to the EV-only group, the groups injected with EV-hydrogel showed an approximately 1.5-time acceleration of the healing of alveolar bone [Figure 9A] and a two-time reduction in the loss of bone, which also matched the reduced amount of cytokine and the increased number of macrophages in anti-inflammatory phenotype for the EV delivery system. In the field of cardiac repair, hUCMSC EVs in functional MMP-responsive polypeptide hydrogels reported by Han and coauthors exhibited an approximately 50% reduction in inflammation, fibrosis and apoptosis, and upregulated angiogenesis for myocardial infarction 28 days after surgery compared to EV-only treatment, as a result of obvious retention of EVs [Figure 4A][122]. A similar result was observed in ischemic limbs parallelly treated with MSC-EVs and MSC-EV-chitosan hydrogel, as shown in Figure 4C[126]. The localized retention of EVs by the material directly led to the reduction of necrotic fibers and inflammatory cells, and improved angiogenesis at both cellular and physiological levels, resulting in optimal muscle recovery after 14-28 days [Figure 9B]. More cases have highlighted the regenerative promise of the administration of EV delivery systems compared to EVs alone[51,56,74,135].
Figure 9. Recovery results of EV delivery hydrogels. (A) Accelerated healing of alveolar bone by dental pulp stem cell-EV chitosan-β-sodium glycerophosphate hydrogels injected within the cavity of periodontitis; (B) reduction of necrotic fibers and inflammatory cells (staining), and improved angiogenesis (live imaging) by MSC-EV-encapsulated amine-aldehyde chitosan hydrogel in hindlimb. Figure 9A adapted with permission[47], Copyright 2020, the authors, published by KeAi Publishing; 9B adapted with permission[126], Copyright 2018, American Chemical Society. EV: Extracellular vesicle; MSC: mesenchymal stem cell.
As for scaffolds, not as many reports compared the results of EV-only and EV-scaffolds groups, since EVs are mostly considered as an enhancement to the functions of scaffolds. Answering the needs for the regeneration of endometrium and fertility, Xin and colleagues loaded hUCMSC-EVs to collagen scaffolds, which could sustain the release over 14 days in an extended burst release manner[133]. The combination of EVs and scaffolds was found to achieve the results of EV-only group in terms of regenerated endometrium thickness and the number of PR+ cells, and have improved effects on the regeneration of glands and reduction of muscular dystrophy after 30 and 60 days of administration [Figure 10A]. Jiang et al. exploited acellular ECM scaffolds laden with Wharton’s jelly MSC-EVs to induce osteochondral regeneration[82]. The EV-scaffolds group was assessed to have promoted ~33% of the recovery of cartilage compared to EV-only group three and six months after transplantation. In the representative case of cell-recruiting mode, the PCL scaffolds immobilized with MSC-EVs through conjugated PEI were used to treat square skin excisional wounds with an area of 225 mm2[92]. After transplantation for two weeks, a lot more newly formed epidermis and the presence of α-smooth muscle actin were found in the tissue using the combinatory material. The EV-scaffolds group exhibited strong function, reaching ~70% of wound closure, while the closure of EV-only group was only 30% and scaffold-only group 20% [Figure 10B].
Figure 10. Recovery results of EV delivery scaffolds. (A) Promoted regeneration of glands and reduction of muscular dystrophy by hUCMSC-EVs laden collagen scaffolds for endometrium injury; (B) faster wound closing (picture) and more formation of new epidermis (staining) by MSC-EVs immobilized on PEI-conjugated PCL scaffolds treating large skin wound. Figure 10A adapted with permission[133], Copyright 2020, Elsevier; 10B adapted with permission under terms of the CC-BY NC license[92], Copyright 2021, the authors, published by American Association for the Advancement of Science. EV: Extracellular vesicle; MSC: mesenchymal stem cell.
With greatly promoted regenerative outcomes resulted from EV delivery systems, the followed key question is whether the in vivo kinetics of delivery agrees with the physiological patterns of stem cells, which is crucial for the regeneration of tissue and the closing of injuries. The tracking of the internalization of EVs delivered by the bulk materials is thus necessary for the recovery procedure of tissue. This is illustrated by the study conducted by Henriques-Antunes et al. on the relationship between the EV release profile and the healing of diabetic chronic wounds[136]. In order to achieve a fully controlled release of EVs in terms of dose and frequency, a photocleavable crosslinker was synthesized to conjugate EVs and thiolated hyaluronic acid to form a hydrogel, so that the delivery can be proceeded by periodical irradiation. It was found that the matching of the release profile of EVs and the dynamics of the regeneration of skin can promote more than 40% healing effect compared to scaffold alone and single administration of EVs [Figure 11]. This single study calls for more investigations based on different kinetics of EV release and the interaction with cells from different tissues.
Figure 11. Schematic illustration of the promoted wound-healing effect of controlled release of EVs from photocleavable hydrogel and the according staining of wounds treated with different approaches at Day five. Adaptations with permission[136], Copyright 2019, American Chemical Society. EVs: Extracellular vesicles.
ADDITIONAL FUNCTIONS OF EV DELIVERY SYSTEMS
In order to enhance the EV delivery systems to be more suitable for specific conditions in diseases or requirements of surgeries, extra functions have been incorporated into the materials through special processing strategies based on the intrinsic properties of the materials or the introduction of specific functional groups/substances to change the property of the material at the molecular level.
In many cases, minimally invasive surgery is required to reduce the suffering of patients. Injectable hydrogels have met such requirements through shear-thinning properties or mixing with double-barrel syringes, but for materials such as scaffolds and photocrosslinkable hydrogels, transplantation is still needed. To compensate for this, the size of these delivery systems must be sufficiently small, i.e., in micrometer class, for direct injection. So far, the primary approach to miniaturizing EV delivery systems is through the formation of emulsion. To fabricate hydrogel microspheres, water-in-oil emulsion is used. This is demonstrated by Liu et al., who developed chitosan microspheres after high-speed homogenization of acidified chitosan-liquid paraffin/petroleum ether/SPAN80 emulsion system[137]. With a diameter of 5 μm, the cationic charged spheres were incubated with anionic rabies virus glycoprotein peptide-modified EVs loaded with reactive oxygen species-responsive curcumin nanoparticles. Although the microspheres were prepared only for better observation of EVs, it suggests the possibility of using microspheres as EV carriers through adsorption and such a concept can be extended in future exploration of EV-based therapies. As for the fabrication of non-water-soluble scaffolds, reverse phase emulsion or double emulsion systems can be applied. Hollow PLGA spheres with a diameter of 50 μm were prepared using water-in-oil-in-water double emulsion systems with the help of sonication. The spheres were eventually surface modified with polydopamine for the attachment of serum-derived EVs[138]. The EV-loaded microspheres were shown to be internalized by DC2.4 and RAW264.7 cells, with the internalization promoted by higher EV loading rates. Such a double emulsion system also allows the use of microfluidic devices for the fabrication of microspheres with more uniform sizes. Through a well-designed PDMS microfluidic chip, Swanson and colleagues were able to fabricate human DPSC-derived EV-encapsulated microspheres with a diameter of around 5 μm, which were later physically adsorbed to poly(L-lactide) (PLLA) scaffolds and transplanted to treat defected calvary of mice[127]. Such a double scaffold system utilized a two-step release, i.e., desorption of microspheres from scaffold and hydrolysis of microsphere to release EVs. The system was found to make the delivery of EVs more linear. It has to be noted that in order not to disrupt the encapsulated EVs by organic solvent, the oil phases of the above-reported spheres were volatile for thorough evaporation and easy collection. Accordingly, dichloromethane and ethyl acetate were used in the above-mentioned two studies. However, the remnant organic solvent is still potentially harmful to tissue and EVs. A replacement of oil phase with air and in situ crosslinking can allow the direct formation of hydrogel microspheres encapsulating EVs within the injured tissue, without the need to remove the organic solvent, as demonstrated by Yao and colleagues[139]. They filled a double-barrel syringe with fibrinogen/EVs mixture as the polymer solution and thrombin solution as the crosslinker, and equipped the tip of the syringe with a spray needle. By spraying out the combined solution, the droplets crosslinked in situ and were sent directly at the myocardial infarction area of a pig model to achieve the purpose of minimum invasive surgery.
Oxidative stress is another frequent problem for tissue regeneration due to a lack of oxygen. Localized supply of oxygen combined with the removal of reactive oxygen species can enhance the result of treatment in addition to the delivery of EVs, especially for chronic diabetic patients. Shiekh and coauthors designed a special polyurethane scaffold for this purpose[99]. The polyurethane was specifically designed to be an antioxidant with vitamin C as one of the monomers to as scavenger of reactive oxygen species [Table 2], while the authors also doped calcium peroxide, which can decompose and produce oxygen when in contact with water, into the polyurethane scaffold for localized delivery of oxygen. The sustained supply of oxygen was confirmed to enhance wound healing of diabetic mice, along with the EVs to stimulate angiogenesis. On Day 14, the antioxidant polyurethane itself could lead to ~98% wound closure, with the non-diabetic wound closure at 90% and diabetic wound closure at only ~80%. With both the oxygen release and EV delivery functions, the wound closing function of the scaffold could reach ~100% after 14 days for both non-diabetic and diabetic mice.
In the field of bone tissue regeneration, specific elements can improve the angiogenesis function of the material so as to stimulate the recovery of injured bones. Li+ has been proven to promote proliferation and osteogenesis. Liu and colleagues doped LiCl in bioglass ceramics to carry BMSC-EVs[112]. The investigation showed that the existence of Li+ in the bioglass could significantly improve angiogenesis through the proliferation of HUVECs both in vitro and in vivo compared to Li+-free bioglass by stimulating the activation of Wnt/β-catenin, AKT and NF-κB signaling pathways. The addition of proangiogenic miRNA-130a in EVs further activated the PTEN/AKT signaling pathway to enhance the proangiogenic capacity of endothelial cells. The synergic effect brought promise to the material for the vascularization of injured bone tissue.
Despite the above designs, however, the incorporation of extra functions has not been applied extensively in the overall studies of EV delivery systems. Along with EVs, the combinatorial and multimodal benefits towards injuries are to be expected in future experiments.
CONCLUSION AND PERSPECTIVES FOR FUTURE DESIGN OF EV DELIVERY SYSTEMS
In this review, we surveyed the EV delivery systems from the viewpoint of the material design at the molecular level, focusing on the relationship of material structures, the corresponding physicochemical properties and the performance of EV delivery. Based on the loading and delivery of EVs, the systems were classified into bulk materials, with crosslinking as the crucial structural parameter, and scaffolds, with porosity as the symbolic structure and surface property as the key parameter to interact with EVs. From the analyses of the according delivery behavior in vitro and in vivo, it can be seen that the delivery can be adjusted in correlation to the changes of material at the molecular level to a certain extent. These all guarantee that the EV delivery systems fill the two “gaps” in the field: one is the successful, localized and sustained release of EVs in the injured tissue; the other is how the material designs can direct the delivery of EVs. From the stand of patients, the use of EV delivery systems can greatly reduce the pain patients receive from otherwise multiple times of systemic, large-dose administration of EVs. This can further lead to cost reduction due to higher efficiency of EV delivery, and better regenerative results due to the various functions different materials can bring about. Therefore, the use of EV delivery systems has shown much promise in promoting EVs as a potential therapeutic approach for tissue regeneration. However, still at its initial stage, the EV delivery systems have missing links within the relationship of material structures, physicochemical properties and the performance of delivery. In order to achieve on-demand control over the delivery behavior through fine-tuning of the materials at the molecular level, we propose and emphasize that EV delivery systems can be further studied in the following directions:
(1) More studies necessary for a better understanding of the impacts of molecular designs on EV delivery
As mentioned in the introduction section, we extracted related information from representative investigations to analyze the relationship between the release profiles in vitro, retention of EVs in vivo and the resulting tissue regeneration. More systematic studies should be conducted on various types of EV delivery systems for the validation of results, as mentioned above. New reports on the behavior of EV delivery systems should be tracked to build a larger and more complete platform for EV delivery systems;
(2) The interaction between the materials and EVs during the EV storage period
The storage period is one of the three periods of EVs in material delivery systems, but is a missing link compared to the loading and delivery of EVs. Although the results of the delivery systems suggest no obvious change in the function of EVs after a short period of storage, no investigation has been made on how the material affects the encapsulated or carried EVs in the long run. For bulk materials, the distribution of crosslinking junctions/salt crystals and of EVs, as mentioned in “IMPACT OF DIFFERENT DESIGNS ON THE LOADING AND DELIVERY OF EVs”, are critical to the release profile of the EVs and the kinetics of interaction with the recipient cells. Similarly, for scaffold materials, the different surface properties, including mechanical properties, hydrophilicity/hydrophobicity, surface charge, and uninvestigated surface topographies, can also affect the loading and delivery performance of the systems. Meanwhile, previous studies[140,141] have shown that surface properties can affect the attached stem cells to secrete EVs with different cargoes. It can be inferred that as the functional replicates of the parental stem cells, different properties of materials, including both bulk ones and scaffolds, may also affect the behavior of EVs encapsulated or attached. On the basis of the understanding of the influence of materials on the EVs, better selection of materials and processing strategies can be made for future development of the delivery systems;
(3) The kinetics of EV delivery and the internalization of cells by the adjustments of material designs
As was shown by Henriques-Antunes et al. on the relationship between the EV release profiles and the healing of diabetic chronic wounds using photo-cleavable crosslinkers in EV delivery hydrogel, the kinetics of EV delivery is crucial to their interactions with cells and wound healing[136]. In order to optimize the delivery behavior of the material, careful detection of the relationship between the rates of delivery and the dosage being internalized is necessary. This can be realized, in resemblance to and more controllable than the photo-cleavable crosslinker, by a smart delivery system to finely adjust the interaction between the material and the EVs for release;
(4) Extension to materials previously investigated in tissue engineering
The current exploitation of materials for EV delivery systems accounts for only a small portion of materials in tissue engineering. Many different materials, especially for drug delivery systems, have been developed and extensively investigated. The EV delivery systems can be adapted from these material systems, which will provide a more diverse choice and functions. Furthermore, the scope of material can be extended to fully synthetic materials. Synthetic free radical polymers, for example, have fine structures and can be accurately controlled with living/controlled polymerization techniques. Through the applications such as ring-opening single unit monomer insertion techniques[142] and anionic ring-opening polymerization (polycarbonate series)[143], the non-degradability of free radical polymers has also been solved for the dynamic building of bioactive polymers. The incorporation of these well-defined polymers into EV delivery systems can be of great promise.
DECLARATIONS
Authors’ contributionsConceptualization, writing-original draft: Chen A
Writing - review & editing: Tian H, Yang N, Zhang Z, Yang GY, Cui W
Conceptualization, supervision, writing - review & editing: Tang Y
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis study is supported by grants from the National Key R&D Program of China #2019YFA0112000 (Tang Y), the National Natural Science Foundation of China (NSFC) projects 82071284 (Tang Y), 81930051 (Cui W), 81771244 (Zhang Z), 81974179 (Zhang Z), the Scientific Research and Innovation Program of Shanghai Education Commission 2019-01-07-00-02-E00064 (Yang GY), Scientific and Technological Innovation Act Program of Shanghai Science and Technology Commission, 20JC1411900 (Yang GY), and GuangCi Professorship Program of Ruijin Hospital Shanghai Jiao Tong University School of Medicine. The International Postdoctoral Exchange Fellowship Program (Talent-Introduction Program) of Ministry of Human Resources and Social Security of the People’s Republic of China is gratefully acknowledged for partial support of this study (Chen A).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585-601.
2. Mulholland EJ, Dunne N, McCarthy HO. MicroRNA as therapeutic targets for chronic wound healing. Mol Ther Nucleic Acids 2017;8:46-55.
3. Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg 1998;176:80S-2S.
4. Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res 2018;22:36.
6. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269-88.
7. Trams EG, Lauter CJ, Norman Salem J, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes 1981;645:63-70.
8. Newton WC, Kim JW, Luo JZQ, Luo L. Stem cell-derived exosomes: a novel vector for tissue repair and diabetic therapy. J Mol Endocrinol 2017;59:R155-65.
9. Al-Sowayan B, Alammari F, Alshareeda A. Preparing the bone tissue regeneration ground by exosomes: from diagnosis to therapy. Molecules 2020;25:4205.
10. Ching RC, Kingham PJ. The role of exosomes in peripheral nerve regeneration. Neural Regen Res 2015;10:743-7.
11. Cooper LF, Ravindran S, Huang CC, Kang M. A role for exosomes in craniofacial tissue engineering and regeneration. Front Physiol 2019;10:1569.
12. Adamiak M, Sahoo S. Exosomes in myocardial repair: advances and challenges in the development of next-generation therapeutics. Mol Ther 2018;26:1635-43.
13. Akbari A, Jabbari N, Sharifi R, et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci 2020;249:117447.
14. Huang J, Xiong J, Yang L, Zhang J, Sun S, Liang Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021;13:8740-50.
15. Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained delivery system for stem cell-derived exosomes. Front Pharmacol 2019;10:1368.
16. Holkar K, Vaidya A, Pethe P, Kale V, Ingavle G. Biomaterials and extracellular vesicles in cell-free therapy for bone repair and regeneration: Future line of treatment in regenerative medicine. Materialia 2020;12:100736.
17. Tsintou M, Dalamagkas K, Moore TL, et al. The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches. Neural Regen Res 2021;16:605-13.
18. Murali VP, Holmes CA. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater 2021;124:88-107.
19. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750.
20. Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998;4:594-600.
21. Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 1997;8:2631-45.
22. Peters PJ, Geuze HJ, Van der Donk HA, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol 1989;19:1469-75.
23. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999;94:3791-9.
24. Fevrier B, Vilette D, Archer F, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 2004;101:9683-8.
25. Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001;7:297-303.
26. Crivelli B, Chlapanidas T, Perteghella S, et al. Italian Mesenchymal Stem Cell Group (GISM). Mesenchymal stem/stromal cell extracellular vesicles: from active principle to next generation drug delivery system. J Control Release 2017;262:104-17.
27. Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med 2017;49:e346.
28. Kim J, Song Y, Park CH, Choi C. Platform technologies and human cell lines for the production of therapeutic exosomes. EVCNA 2021; doi: 10.20517/evcna.2020.01.
29. Liu M, Sun Y, Zhang Q. Emerging role of extracellular vesicles in bone remodeling. J Dent Res 2018;97:859-68.
30. Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 2011;10:117.
31. Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics 2010;9:1085-99.
32. Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res 2013;319:2113-23.
33. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 2020;15:6917-34.
34. la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T, Kussmann M. “Exosomics”-a review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet 2018;9:92.
35. Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014;8:483-94.
36. Heusermann W, Hean J, Trojer D, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol 2016;213:173-84.
37. Ma Z, Mao Z, Gao C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf B Biointerfaces 2007;60:137-57.
38. Banerjee A, Arha M, Choudhary S, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009;30:4695-9.
39. Chatterjee K, Lin-Gibson S, Wallace WE, et al. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials 2010;31:5051-62.
40. Cai S, Wu C, Yang W, Liang W, Yu H, Liu L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnology Reviews 2020;9:971-89.
41. Metwally S, Stachewicz U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater Sci Eng C Mater Biol Appl 2019;104:109883.
42. Sivashanmugam A, Arun Kumar R, Vishnu Priya M, Nair SV, Jayakumar R. An overview of injectable polymeric hydrogels for tissue engineering. European Polymer Journal 2015;72:543-65.
43. Vernerey FJ, Lalitha Sridhar S, Muralidharan A, Bryant SJ. Mechanics of 3D cell-hydrogel interactions: experiments, models, and mechanisms. Chem Rev 2021;121:11085-148.
44. Vedadghavami A, Minooei F, Mohammadi MH, et al. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater 2017;62:42-63.
45. Johns MA, Bae Y, Guimarães FEG, et al. Predicting ligand-free cell attachment on next-generation cellulose-chitosan hydrogels. ACS Omega 2018;3:937-45.
46. Kazi GAS, Yamanaka T, Osamu Y. Chitosan coating an efficient approach to improve the substrate surface for in vitro culture system. J Electrochem Soc 2019;166:B3025-30.
47. Shen Z, Kuang S, Zhang Y, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater 2020;5:1113-26.
48. Wang M, Wang C, Chen M, et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano 2019;13:10279-93.
49. Zhao X, Liu Y, Jia P, et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res Ther 2021;12:196.
50. Zhang FX, Liu P, Ding W, et al. Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021;278:121169.
51. Wang C, Liang C, Wang R, et al. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater Sci 2019;8:313-24.
52. Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J Mol Histol 2020;51:251-63.
53. Zhang Y, Xie Y, Hao Z, et al. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces 2021;13:18472-87.
54. Xin L, Wei C, Tong X, et al. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioact Mater 2022;12:107-19.
55. Mol EA, Lei Z, Roefs MT, et al. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthc Mater 2019;8:e1900847.
56. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine 2020;15:5911-26.
57. Fuhrmann G, Chandrawati R, Parmar PA, et al. Engineering extracellular vesicles with the tools of enzyme prodrug therapy. Adv Mater 2018;30:e1706616.
58. Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl 2021;120:111671.
59. Zhang C, Shang Y, Chen X, et al. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano 2020;14:12133-47.
60. Zhu D, Li Z, Huang K, Caranasos TG, Rossi JS, Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat Commun 2021;12:1412.
61. Shen Y, Xu G, Huang H, et al. Sequential release of small extracellular vesicles from bilayered thiolated alginate/polyethylene glycol diacrylate hydrogels for scarless wound healing. ACS Nano 2021;15:6352-68.
62. Mardpour S, Ghanian MH, Sadeghi-Abandansari H, et al. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl Mater Interfaces 2019;11:37421-33.
63. Wang C, Wang M, Xu T, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019;9:65-76.
64. Liu X, Yang Y, Li Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017;9:4430-8.
65. Wang L, Wang J, Zhou X, et al. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol 2020;8:564731.
66. Yang S, Zhu B, Yin P, et al. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng 2020;6:1590-602.
67. Yan HC, Yu TT, Li J, et al. The delivery of extracellular vesicles loaded in biomaterial scaffolds for bone regeneration. Front Bioeng Biotechnol 2020;8:1015.
68. Jiang Y, Wang R, Wang C, et al. Brain microenvironment responsive and pro-angiogenic extracellular vesicle-hydrogel for promoting neurobehavioral recovery in type 2 diabetic mice after stroke. Adv Healthc Mater 2022:e2201150.
69. Yu H, Cheng J, Shi W, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater 2020;106:328-41.
70. Lenzini S, Bargi R, Chung G, Shin JW. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol 2020;15:217-23.
71. Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013;14:4125-34.
72. Chen CW, Wang LL, Zaman S, et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res 2018;114:1029-40.
73. Li Q, Gong S, Yao W, et al. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Deliv 2021;28:884-93.
74. Guan P, Liu C, Xie D, et al. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact Mater 2022;10:145-58.
75. Swanson WB, Gong T, Zhang Z, et al. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J Control Release 2020;324:679-94.
76. Boffito M, Bernardi E, Sartori S, Ciardelli G, Sassi MP. A mechanical characterization of polymer scaffolds and films at the macroscale and nanoscale. J Biomed Mater Res A 2015;103:162-9.
77. Wang B, Li P, Shangguan L, et al. A novel bacterial cellulose membrane immobilized with human umbilical cord mesenchymal stem cells-derived exosome prevents epidural fibrosis. Int J Nanomedicine 2018;13:5257-73.
78. Prasadh S, Wong RCW. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Science International 2018;15:48-55.
79. Baker SC, Rohman G, Southgate J, Cameron NR. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials 2009;30:1321-8.
80. Zhu X, Cui W, Li X, Jin Y. Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 2008;9:1795-801.
81. Xie H, Wang Z, Zhang L, et al. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci Rep 2017;7:45622.
82. Jiang S, Tian G, Yang Z, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater 2021;6:2711-28.
83. Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg 2012;130:232S-41S.
84. Zhang L, Fan C, Hao W, et al. NSCs Migration promoted and drug delivered exosomes-collagen scaffold via a bio-specific peptide for one-step spinal cord injury repair. Adv Healthc Mater 2021;10:e2001896.
85. Chew JRJ, Chuah SJ, Teo KYW, et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater 2019;89:252-64.
87. Kim D, Lee S, Kim M, Jeong Y, Lee S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem Eng J 2021;406:127080.
88. Galeotti F, Andicsova A, Bertini F, et al. A versatile click-grafting approach to surface modification of silk fibroin films. J Mater Sci 2013;48:7004-10.
89. Ybañez MG, Camacho DH. Designing hydrophobic bacterial cellulose film composites assisted by sound waves. RSC Advances 2021;11:32873-83.
90. Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microRNA-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med 2017;6:736-47.
91. Shi Q, Qian Z, Liu D, et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol 2017;8:904.
92. Su N, Hao Y, Wang F, Hou W, Chen H, Luo Y. Mesenchymal stromal exosome-functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci Adv 2021;7:eabf7207.
93. Wei Y, Wu Y, Zhao R, et al. MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials 2019;204:13-24.
94. Wurth JJ, Blumenthal NR, Shastri VP. Hydrophilization of poly(caprolactone) copolymers through introduction of oligo(ethylene glycol) moieties. PLoS ONE 2014;9:e99157.
95. Gandolfi MG, Gardin C, Zamparini F, et al. Mineral-doped poly(L-Lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomaterials 2020;10:432.
96. Hendrick E, Frey M. Increasing surface hydrophilicity in poly(lactic acid) electrospun fibers by addition of Pla-B-Peg co-polymers. J Eng Fiber Fabr 2014;9:155892501400900219.
97. Li W, Liu Y, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces 2018;10:5240-54.
98. Zhang Z, Wang X, Zhu R, et al. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polymer Science Series B 2016;58:720-29.
99. Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials 2020;249:120020.
100. Shiekh PA, Singh A, Kumar A. Data supporting exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Data Brief 2020;31:105671.
101. Fan L, Guan P, Xiao C, et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact Mater 2021;6:2754-66.
102. Ha SW, Kirch M, Birchler F, et al. Surface activation of polyetheretherketone (peek) and formation of calcium phosphate coatings by precipitation. J Mater Sci Mater Med 1997;8:683-90.
103. Lee JH, Khang G, Lee JW, Lee HB. Interaction of different types of cells on polymer surfaces with wettability gradient. J Colloid Interface Sci 1998;205:323-30.
104. Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019;9:2439-59.
105. Huang CC, Kang M, Shirazi S, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater 2021;126:199-210.
106. Xiao D, Yang M, Zhang M, et al. MicroRNA-126 from stem cell extracellular vesicles encapsulated in a tri-layer hydrogel scaffold promotes bladder angiogenesis by activating CXCR4/SDF-1α pathway. Chem Eng J 2021;425:131624.
107. Sun Y, Zhang B, Zhai D, Wu C. Three-dimensional printing of bioceramic-induced macrophage exosomes: immunomodulation and osteogenesis/angiogenesis. NPG Asia Mater 2021:13.
108. Zamiri A, De S. Mechanical properties of hydroxyapatite single crystals from nanoindentation data. J Mech Behav Biomed Mater 2011;4:146-52.
109. Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther 2016;7:136.
110. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater 2019;86:480-92.
111. Zhai M, Zhu Y, Yang M, Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv Sci (Weinh) 2020;7:2001334.
112. Liu L, Liu Y, Feng C, et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials 2019;192:523-36.
113. Qayoom I, Teotia AK, Kumar A. Nanohydroxyapatite based ceramic carrier promotes bone formation in a femoral neck canal defect in osteoporotic rats. Biomacromolecules 2020;21:328-37.
115. Liu A, Lin D, Zhao H, et al. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021;272:120718.
116. Liu L, Yu F, Li L, et al. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater 2021;119:444-57.
117. Chen S, Tang Y, Liu Y, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif 2019;52:e12669.
118. Gandolfi MG, Zamparini F, Degli Esposti M, et al. Polylactic acid-based porous scaffolds doped with calcium silicate and dicalcium phosphate dihydrate designed for biomedical application. Mater Sci Eng C Mater Biol Appl 2018;82:163-81.
119. Shafei S, Khanmohammadi M, Heidari R, et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res A 2020;108:545-56.
120. Li L, Zhang Y, Mu J, et al. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett 2020;20:4298-305.
121. Han C, Zhou J, Liu B, et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater Sci Eng C Mater Biol Appl 2019;99:322-32.
122. Han C, Zhou J, Liang C, et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci 2019;7:2920-33.
123. Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release 2001;73:121-36.
124. Lin J, Wang Z, Huang J, et al. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small 2021;17:e2007235.
126. Zhang K, Zhao X, Chen X, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces 2018;10:30081-91.
127. Swanson WB, Zhang Z, Xiu K, et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater 2020;118:215-32.
128. Wu J, Chen L, Wang R, et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng 2019;5:3561-71.
129. Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int J Biol Macromol 2019;121:38-54.
130. Yang Y, Shen G, Wang H, et al. Interferometric plasmonic imaging and detection of single exosomes. Proc Natl Acad Sci U S A 2018;115:10275-80.
131. Wan Y, Liu X, Liu P, Zhao L, Zou W. Optimization adsorption of norfloxacin onto polydopamine microspheres from aqueous solution: Kinetic, equilibrium and adsorption mechanism studies. Sci Total Environ 2018;639:428-37.
132. Lazar S, Mor S, Chen J, Hao D, Wang A. Bioengineered extracellular vesicle-loaded bioscaffolds for therapeutic applications in regenerative medicine. Extracell Vesicles Circ Nucl Acids 2021;2:175-8.
133. Xin L, Lin X, Zhou F, et al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater 2020;113:252-66.
134. Hao D, Swindell HS, Ramasubramanian L, et al. Extracellular matrix mimicking nanofibrous scaffolds modified with mesenchymal stem cell-derived extracellular vesicles for improved vascularization. Front Bioeng Biotechnol 2020;8:633.
135. Xu N, Wang L, Guan J, et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol 2018;117:102-7.
136. Henriques-Antunes H, Cardoso RMS, Zonari A, et al. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano 2019;13:8694-707.
137. Liu L, Li Y, Peng H, et al. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson’s disease. Sci Adv 2020;6:eaba3967.
138. You G, Kim Y, Lee JH, Song J, Mok H. Exosome-modified PLGA microspheres for improved internalization into dendritic cells and macrophages. Biotechnol Bioproc E 2020;25:521-7.
139. Yao J, Huang K, Zhu D, et al. A minimally invasive exosome spray repairs heart after myocardial infarction. ACS Nano ;2021:11099-111.
140. Liu M, Wang D, Gu S, et al. Micro/nano materials regulate cell morphology and intercellular communication by extracellular vesicles. Acta Biomater 2021;124:130-8.
141. Liang J, Gu S, Mao X, et al. Endothelial cell morphology regulates inflammatory cells through microRNA transferred by extracellular vesicles. Front Bioeng Biotechnol 2020;8:369.
142. Yang Y, Yu K, Liu S, et al. Radical ring-opening single unit monomer insertion: an approach to degradable and biocompatible sequence-defined oligomers. Macromolecules 2021;54:10923-30.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chen A, Tian H, Yang N, Zhang Z, Yang GY, Cui W, Tang Y. Towards extracellular vesicle delivery systems for tissue regeneration: material design at the molecular level. Extracell Vesicles Circ Nucleic Acids 2022;3:323-56. http://dx.doi.org/10.20517/evcna.2022.37
AMA Style
Chen A, Tian H, Yang N, Zhang Z, Yang GY, Cui W, Tang Y. Towards extracellular vesicle delivery systems for tissue regeneration: material design at the molecular level. Extracellular Vesicles and Circulating Nucleic Acids. 2022; 3(4): 323-56. http://dx.doi.org/10.20517/evcna.2022.37
Chicago/Turabian Style
Chen, Ao, Hengli Tian, Nana Yang, Zhijun Zhang, Guo-Yuan Yang, Wenguo Cui, Yaohui Tang. 2022. "Towards extracellular vesicle delivery systems for tissue regeneration: material design at the molecular level" Extracellular Vesicles and Circulating Nucleic Acids. 3, no.4: 323-56. http://dx.doi.org/10.20517/evcna.2022.37
ACS Style
Chen, A.; Tian H.; Yang N.; Zhang Z.; Yang G.Y.; Cui W.; Tang Y. Towards extracellular vesicle delivery systems for tissue regeneration: material design at the molecular level. Extracell. Vesicles. Circ. Nucleic. Acids. 2022, 3, 323-56. http://dx.doi.org/10.20517/evcna.2022.37
About This Article
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks


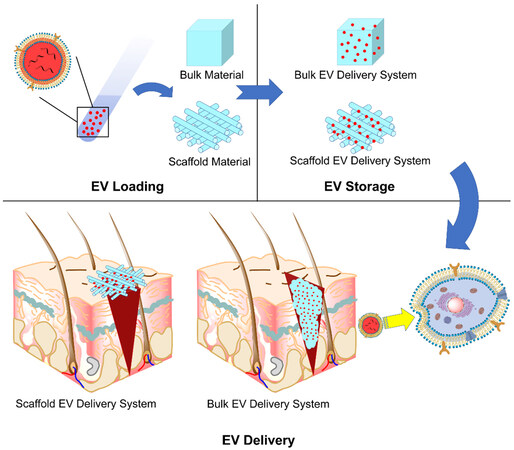
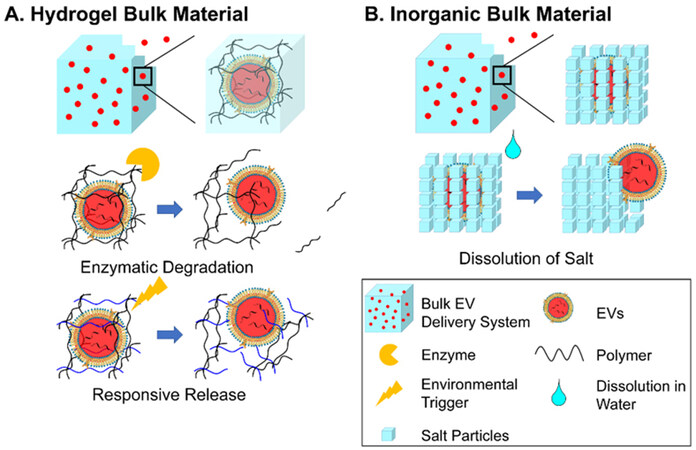
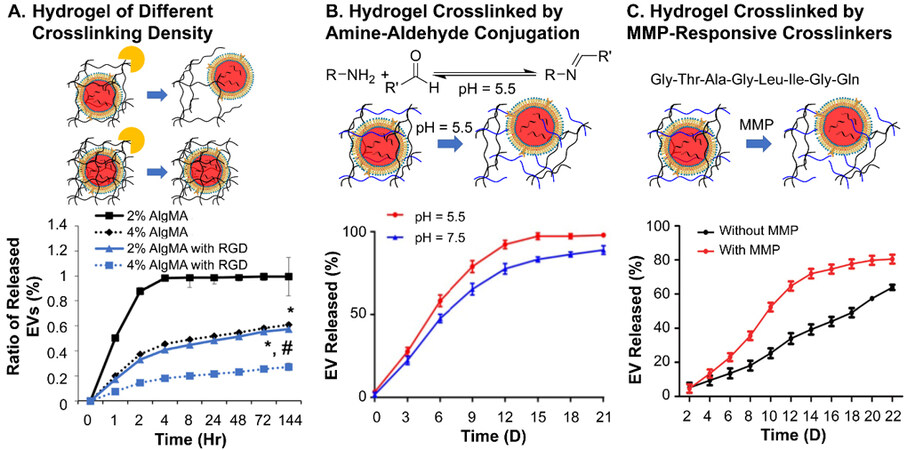
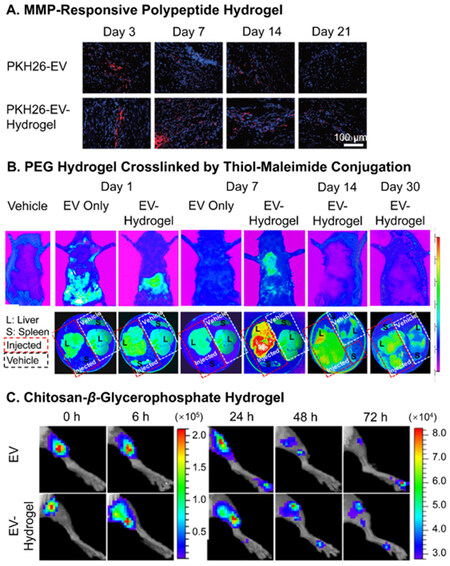
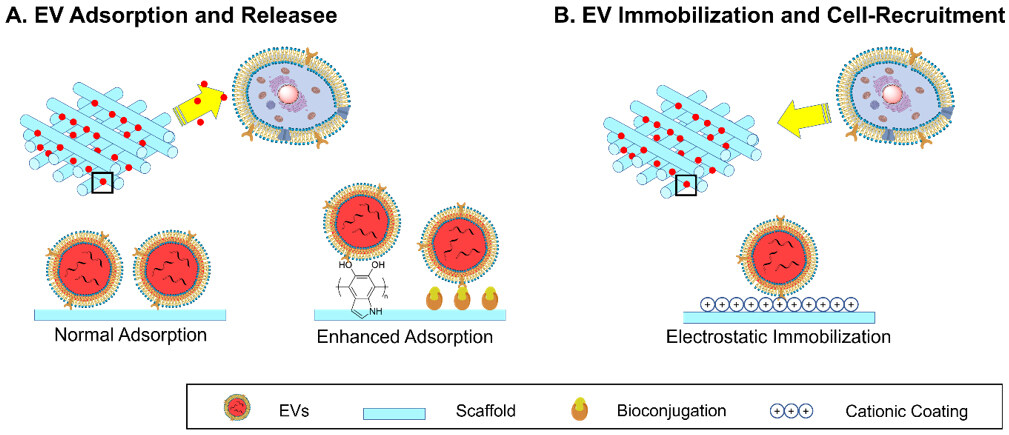
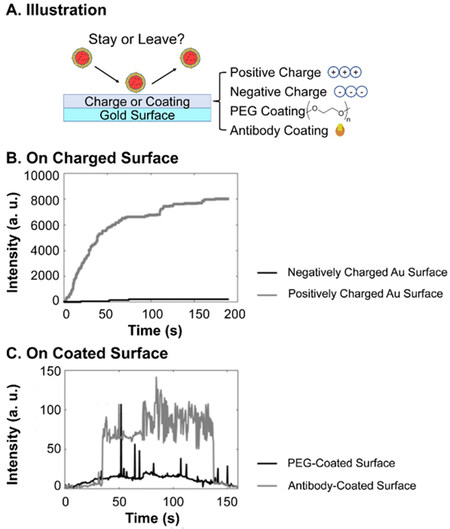
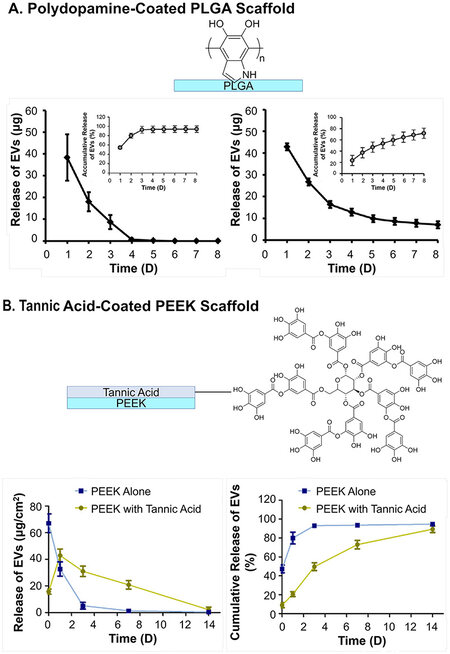
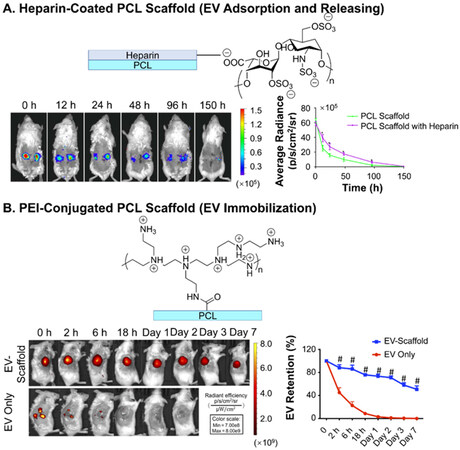
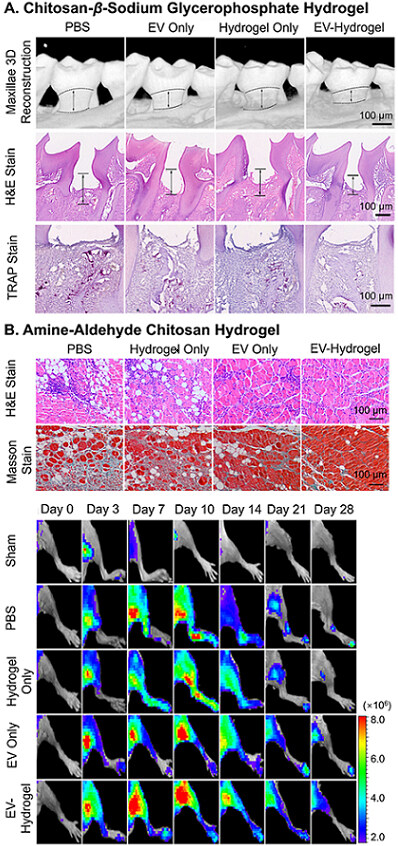
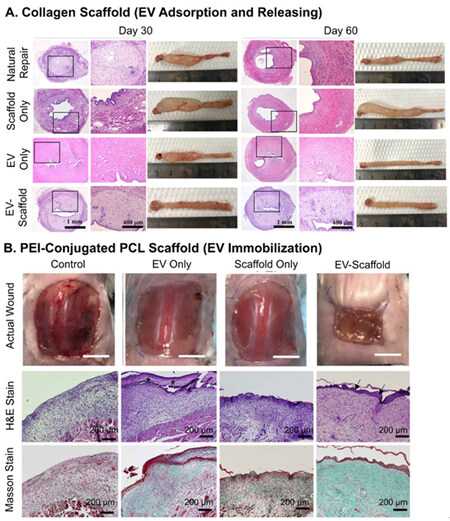
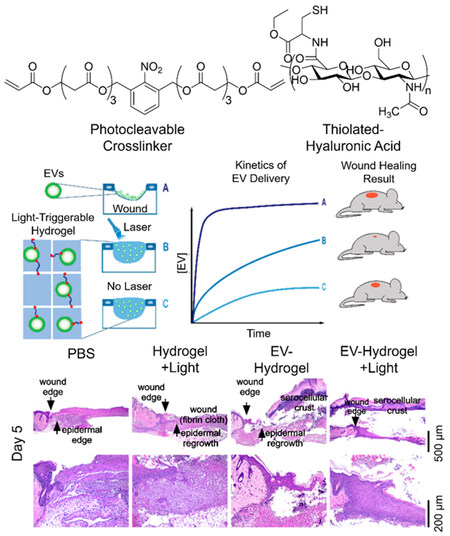











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.