Early and mid-term results in patients undergoing primary CABG in comparison with patients with PCI prior to CABG
Abstract
Aim: We evaluated the impact of prior percutaneous coronary intervention (PCI) on early and mid-term results in patients undergoing coronary artery bypass grafting (CABG).
Methods: Between 2015 and 2020, 938 consecutive patients (mean age 67.4 ± 9.11 years) underwent CABG with prior PCI (n = 121) or primary CABG (n = 817). The mean follow-up was 37 ± 25 (median 36) months. Kaplan-Meier estimates were used to assess survival rates, while Logistic and Cox model analysis regressions assessed the risk of prior PCI and other variables.
Results: Six-year survival including in-hospital mortality was 79% ± 6% in CABG with prior-PCI patients vs.
Conclusion: Patients undergoing CABG after prior PCI have worse perioperative outcomes. Mid-term reduced survival in the prior-PCI patients is mainly due to the concomitant presence of worse clinical presentation and increased comorbidity. Freedom from cardiac death is comparable and satisfactory in both cohorts, highlighting the positive protective effect of CABG over time.
Keywords
INTRODUCTION
Percutaneous coronary intervention (PCI) has become an important method of coronary revascularization globally, especially as a first strategy if the atherosclerotic coronary artery disease is not very extensive or does not involve the proximal ostia of the coronary arteries. Moreover, the incidence of restenosis after PCI decreased from 32%-55% in the pre-stent era to 17%-41% in the bare metal stent era and now < 10% in the current drug-eluting stent era[1].
However, the extensive use of PCI has resulted in an increase in coronary artery bypass grafting (CABG) patients previously treated with PCI[1-3], with an incidence between 10% and 20% of patients subsequently undergoing surgical revascularization.
Typically, patients with previous PCI undergo CABG either because in-stent restenosis is present, the progression of atherosclerosis in native coronary arteries, or for both causes of the disease. Sometimes, however, the need for urgent or emergency surgical revascularization in patients who have previously undergone PCI is due to the rapid progression of atherosclerotic disease into the lumen of the stent placed in coronary vessels or the presence of in-stent thrombosis causing acute syndromes, i.e., unstable angina, non-ST (segment S-T of ECG) myocardial infarction, or ST myocardial infarction[4-5].
Some retrospective studies and meta-analyses have shown that PCI prior to CABG neither increases the surgical risk of CABG nor causes worse late outcomes during follow-up[2,3,6]. On the contrary, others have shown an increase in both mortality and intraoperative complications[7-8], as well as a worse late outcome after CABG[9].
We aimed to retrospectively analyze the cohort of all consecutive patients undergoing isolated CABG over five years of a single-center surgical activity in order to evaluate the influence of prior PCI to CABG on early and medium-term outcomes and analyze the clinical presentation of patients previously subjected to PCI in comparison to those undergoing primary CABG.
We also investigated several risk factors for short-term mortality and other variables potentially influencing early and mid-term outcomes, i.e., cumulative survival and freedom from all-cause late death, cardiac death, and adverse cardiovascular events during a 6-year period of follow-up.
METHODS
Between January 2015 and December 2020, at the Cardiac Surgery Division of the Tor Vergata University Polyclinic, 938 consecutive patients (793 males, 145 females; mean age 67.4 ± 9.1 years) underwent isolated CABG, of whom 121 patients (13%) had undergone prior PCI with drug-eluting stents for the majority of patients or with bare-metal stents. The average time of PCI was of 73 ± 71 months before CABG. Preoperative mean value of EuroSCORE-2 was 2.85 ± 3.41%; 823 patients (87.7%) were at low risk EuroSCORE-2, 92 (9.8%) at medium risk, and 23 (2.5%) at high risk. At in-hospital admission, the clinical presentation was stable angina in 354 patients (37.7%), unstable angina in 324 patients (34.5%), and recent (< 15 days) non-ST myocardial infarction and ST myocardial infarction in 190 (20.3%) and 70 cases (7.5%), respectively. Indication for CABG surgery was given electively in 214 patients (22.8%), urgency in 710 patients (75.7%), and emergency in 14 cases (1.5%). Mean value of the left ventricular ejection fraction was 0.52 (median 0.55) ± 0.09. In total, 343 patients (36.5%) were affected by diabetes mellitus, of whom 280 were insulin-dependent.
The current study considered all consecutive patients undergoing CABG as an isolated procedure. Other CABG procedures performed in association with aortic or mitral valve repair or replacement, or in association with ascending aorta repair, were excluded. Patients who underwent prior PCI less than seven days before CABG were not included in the study.
The study was approved by the Institutional Review Board of the Tor Vergata Polyclinic, Registry No. 44/22. All patients gave informed surgical consent. The study was designated as a retrospective cohort.
Definitions and data analysis
Operative or in-hospital mortality included deaths occurring in the hospital after an operation or within 30 days after discharge. Perioperative myocardial infarction was defined as an increase of serum troponin I levels > 10 ng/mL associated with an increase of creatine kinase muscle-brain (CK-MB) enzyme > 10% of the total CK enzyme and the onset of ECG anomalies. Postoperative low cardiac output syndrome was so defined when the cardiac index value was < 2.0 L/min/m2 with or without renal impairment requiring inotropic support by means of the infusion of epinephrine, norepinephrine, or levosimendan or requiring intra-aortic balloon pump insertion, for a postoperative period greater than 48 h. Acute kidney injury was defined as a two-fold increase of preoperative serum creatinine level or oliguria, requiring continuous
Follow-up clinical evaluation was performed with an outpatient visit to the patient and/or by telephone interview at 38 ± 25 (median 36, range 6-85) months after CABG surgery. In particular, the following adverse cardiac and cardiovascular events were evaluated over time: late death from all causes, cardiac death, major adverse cardiovascular events (MACE), death from cardiac causes, myocardial infarction, and need for new myocardial revascularization.
Surgical techniques
Surgery was performed through a complete sternotomy in all patients. On-pump CABG was traditionally performed by means of cardiopulmonary bypass and ascending aorta cross-clamping. Cardiac arrest was achieved using intermittent antegrade warm blood cardioplegia, 600 mL as the first dose followed by 400 mL doses administered every 20-25 min, or with the use of St. Thomas cold crystalloid solution, 10 mL/kg as the first dose, followed by 5 mL/kg doses administered every 30-35 min[10].
In patients operated on beating heart, distal perfusion of the coronary arteries was maintained after arteriotomy of the coronary arteries by means of intravascular shunts 1.0, 1.5, and 2.0 mm (Clearview, Medtronic Inc., Minneapolis, Minnesota, US). Monitoring of cardiac function was obtained with
In most cases, coronary artery bypass surgery was performed using the left internal mammary artery as a graft to the left anterior descending (LAD) artery in association with saphenous vein grafts, or as single and less frequently in the “Y” composition, for the revascularization of the right coronary artery and/or to the left circumflex artery branches.
The use of the autologous saphenous vein alone to perform CABG for coronary vessels without the internal mammary artery harvesting was reserved for patients suffering from severe pulmonary disease, i.e., in the presence of diffuse bronchiectasis and emphysematous bubbles, or proximal stenosis of the subclavian artery, or when hemodynamic instability was present at the anesthetic induction.
Statistical analysis
Statistical analysis was performed with the use of Stat View 4.5 (SAS Institute Inc., Abacus Concepts, Berkeley, CA). Contingency table raw data, with the use of Chi-Squared and Fisher’s exact tests for categorical variables and the unpaired Student’s t-test for continuous variables, were calculated to perform the comparisons of patients with prior PCI with those operated on primary CABG. Preoperative analyzed variables included age, sex, EuroSCORE-2, clinical presentation (i.e., previous myocardial infarction, stable angina or coronary artery syndrome, and left ventricular ejection fraction), body mass index, comorbidity (i.e., smoking habit, diabetes mellitus, hyperlipidemia, hypertension, chronic obstructive pulmonary disease, chronic renal dysfunction, and peripheral carotid and vascular disease), an indication to CABG surgery (i.e., elective, urgent, or emergency), and prior PCI vs. primary CABG.
In the prior-PCI group, we analyzed indications of that time, i.e., culprit lesion, non-ST segment Elevation (STE), STE myocardial infarction, number of the stented coronary vessel(s) (i.e., 1 vs. 2-3), and localization of the stent, i.e., proximal circumflex artery-first obtuse marginal branch and distal branches, proximal LAD artery and its middle-distal tract, and proximal right coronary artery and middle-distal tract-posterior descending artery. Intraoperative analyzed variables included the number of grafts per patient, cardiopulmonary bypass and aortic cross-clamp times, the use of internal mammary artery graft, and the type of cardioplegia delivered.
Univariate analysis of preoperative and intraoperative variables considered as potential risk factors of operative mortality was performed; the variables that reached a P value < 0.1 were included in the multivariate Logistic regression analysis.
Cumulative survival including both in-hospital and late mortality and freedom from all-cause late death, late cardiac death, and MACE were calculated by means of the Kaplan-Meier method, and were expressed as mean values of percentage ± standard deviation. The Mantel-Cox Log-rank and Breslow-Gehan-Wilcoxon rank tests were used to compare the curves of freedom from events, i.e., among patients undergoing prior PCI to CABG or primary CABG. The Cox proportional regression analysis was used to evaluate the influence of the calculated variables on time to death and adverse events. All continuous values were expressed as mean ± standard deviation. P values < 0.05 were considered statistically significant.
RESULTS
Main different preoperative and intraoperative characteristics and variables of the patients with prior PCI to CABG (n = 121) and those with primary CABG patients (n = 817) are reported in Tables 1 and 2. The main preoperative differences among the two groups of patients were found to be the worse clinical presentation at the time of surgery and the greater incidence of comorbidity in prior-PCI patients (P < 0.05, for all comparisons) [Table 1]. The LAD territory was revascularized in all patient populations. Overall operative mortality was 2.2% (n = 21/938 patients). As compared with primary CABG, patients who underwent prior PCI experienced higher in-hospital mortality (6.6% vs. 1.6%) and higher incidence of postoperative complications, i.e., low cardiac output syndrome (8.3% vs. 2.7%) and acute renal impairment (9.1% vs. 3.1%) (P < 0.05, for all comparisons) [Table 2]. There were no statistically significant differences among the intraoperative analyzed variables.
Preoperative characteristics of prior-PCI vs. primary-CABG patients
| Characteristics | prior-PCI (n = 121) | primary CABG (n = 817) | P-value |
| Left ventricular ejection fraction | 0.50 ± 9.6 | 0.53 ± 8.6 | 0.01 |
| Previous MI (> 60 days), n (%) | 66 (55) | 135 (17) | < 0.0001 |
| Clinical presentation, n (%): Stable angina Unstable angina | 43 (36) 30 (25) | 311 (38) 294 (36) | < 0.0001 |
| Non-STE MI STE MI | 26 (21) 22 (18) | 164 (20) 48 (6) | |
| Indication to CABG, n (%): Elective Urgent | 28 (23) 90 (74) | 186 (23) 620 (76) | 0.62 |
| Emergency | 3 (3) | 11 (1) | |
| Diabetes mellitus, n (%) | 59 (49) | 254 (31) | 0.01 |
| Euroscore-2, n (%): Low risk Medium risk | 97 (80) 23 (19) | 726 (89) 69 (8) | 0.001 |
| High risk | 1 (1) | 22 (3) | |
| Severe chronic renal dysfunction, n (%) | 18 (15) | 63 (7.8) | 0.01 |
| Moderate chronic renal dysfunction, n (%) | 40 (33) | 251 (31) | 0.81 |
Intraoperative and postoperative analyzed variables
| Variables | Prior-PCI (n = 121) | Primary CABG (n = 817) | P-value |
| Cardiopulmonary bypass, minutes | 106.1 ± 85 | 97.8 ± 57 | 0.21 |
| Aortic cross-clamp, minutes | 63.6 ± 39 | 57.2 ± 25 | 0.06 |
| No. grafts per-patient, mean v. | 2.6 ± 0.8 | 2.8 ± 0.8 | 0.07 |
| Internal mammary artery use, n (%) | 110 (91) | 752 (92) | > 0.90 |
| Bilateral internal mammary artery use, n (%) | 6 (5.0) | 40 (4.9) | > 0.90 |
| Off-pump CABG, n (%) | 12 (9.9) | 71 (8.7) | 0.66 |
| Operative mortality, n (%) | 8 (6.6) | 13 (1.6) | 0.005 |
| Postoperative low cardiac output syndrome with or without perioperative MI, n (%) | 10 (8.3) | 22 (2.7) | 0.02 |
| Acute renal dysfunction, n (%) | 11 (9.1) | 25 (3.1) | 0.01 |
| Respiratory failure, n (%) | 17 (14) | 99 (12) | > 0.99 |
| Surgical re-exploration for bleeding, n (%) | 2 (1.7) | 10 (1.2) | 0.67 |
| Neurological complications, n (%) | 2 (1.7) | 8 (1.0) | > 0.99 |
Independent risk factors of operative mortality were the emergency CABG (P < 0.001), the preoperative severe renal dysfunction (P = 0.02), prior PCI (P = 0.01), its localization on the proximal circumflex artery at the first obtuse marginal branch (P < 0.001), and more than one stented coronary vessels at the time of prior PCI (P = 0.05) [Table 3].
Independent risk factors of operative mortality (Logistic regression analysis)
| Variables | Hazard ratio | 95%CI | P-value |
| Emergency vs. urgent or elective CABG | 16.5 | 3.31-82.0 | < 0.001 |
| Severe chronic renal dysfunction | 0.23 | 0.07-0.77 | 0.02 |
| Prior PCI vs. primary CABG | 4.23 | 1.39-12.6 | 0.01 |
| Prior PCI on proximal circumflex artery-obtuse marginal branch | 0.04 | 0.01-0.25 | < 0.001 |
| No. stented coronary vessels at the time of prior PCI (≥ 2 vessels vs. 1 vessel) | 16.9 | 1.91-316 | 0.05 |
| Prior PCI on proximal left anterior descending artery | 0.11 | ||
| Prior PCI on proximal right coronary artery | 0.13 | ||
| Left ventricular ejection fraction (0.46 vs. 0.52) | 0.18 |
In greater detail, the patients operated on in emergency had a rather compromised clinical profile, both for the higher incidence of comorbidity, i.e., severe chronic renal failure (28.5% vs. 7.5% and 6.5% in urgent and elective cases, respectively; P = 0.01), and for a more severe advanced ischemic heart disease due to a lower preoperative value of the left ventricular ejection fraction (0.46 vs. 0.52 and 0.54, respectively; P < 0.05). The causes of in-hospital death observed in the two groups of patients are reported in Table 4.
Causes of in-hospital death
| Cause | Prior-PCI (n = 8) | Primary CABG (n = 13) |
| Cardiogenic shock, n | 5 | - |
| Sudden cardiac death, n | 1 | 2 |
| Low output cardiac syndrome, n | 1 | 2 |
| Multiple organ failure, n | 1 | 3 |
| Primary respiratory failure, n | - | 2 |
| Post-infarction heart rupture | - | 1 |
| Septic shock, n | - | 1 |
| Acute mesenteric ischemia, n | - | 1 |
| Acute kidney dysfunction, n | - | 1 |
Cumulative survival, freedom from late death, cardiac death and major adverse cardiovascular events
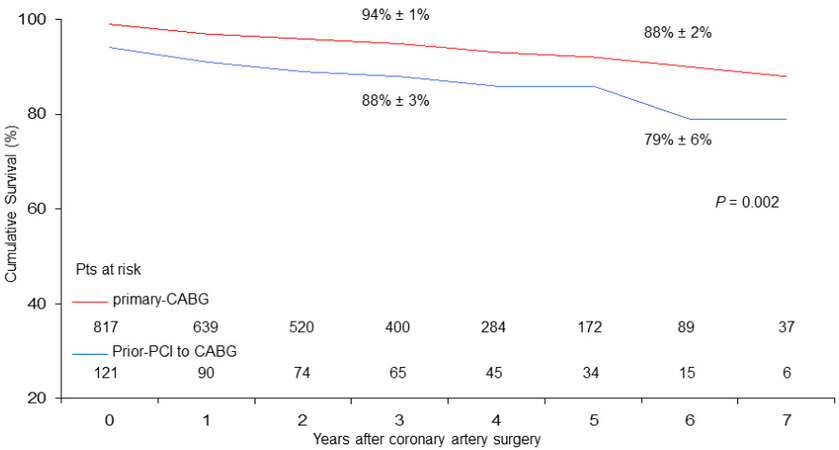
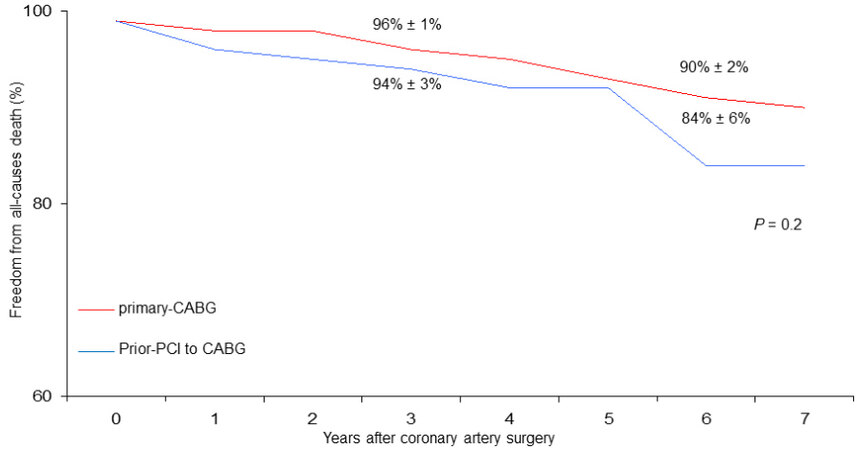
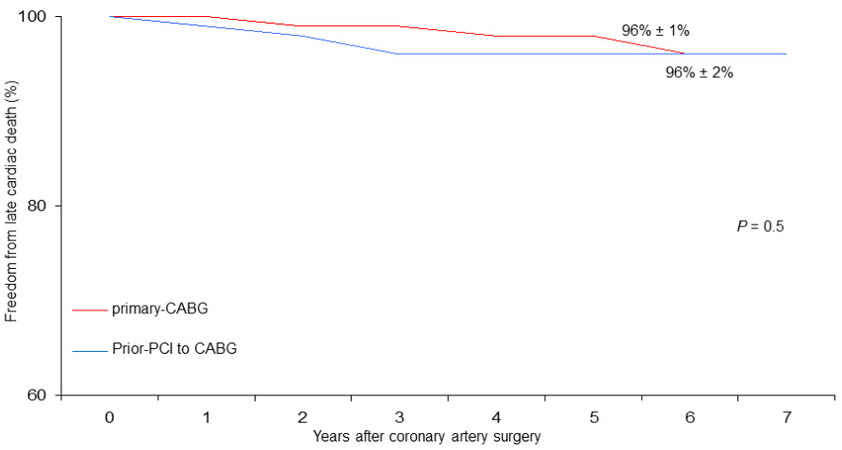
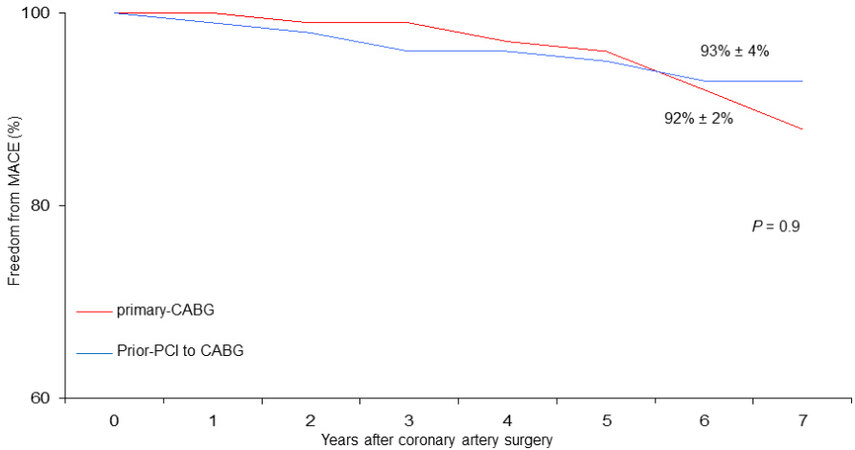
At six years, cumulative survival including in-hospital mortality was 79% ± 6% in CABG with prior-PCI patients and 88 ± 2% in those who underwent primary CABG (P = 0.002) [Figure 1]. During follow-up, there were 46 late deaths out of 917 patients surviving after surgery (5%); 15 of the deaths (33%) were due to cardiac causes. The rate of MACE was 4% (37 patients). At six years, the Rank tests showed that, as compared with primary CABG, in prior-PCI patients freed from late all-cause death, cardiac death, and MACE were 84% ± 6%, 96% ± 2%, and 93% ± 4% vs. 90% ± 2%, 96% ± 1%, and 92% ± 2%, respectively
Figure 1. Cumulative survival (vertical axis) in the years after surgery (horizontal axis) in patients undergoing coronary surgery with prior PCI to CABG vs. primary CABG (Rank tests analysis).
Figure 2. Freedom from late all-cause death in patients undergoing coronary surgery with prior PCI to CABG vs. primary CABG.
Figure 3. Freedom from late cardiac death in patients undergoing coronary surgery with prior PCI to CABG vs. primary CABG.
Figure 4. Freedom from MACE (major adverse cardiovascular events) in patients undergoing coronary surgery with prior PCI to CABG vs. primary CABG.
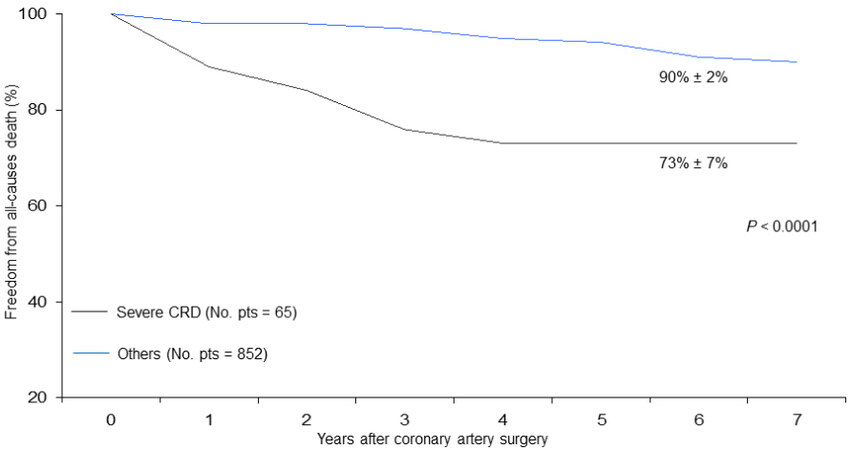
Figure 5. Freedom from late all-cause death in patients undergoing coronary surgery with or without a severe degree of preoperative renal dysfunction.
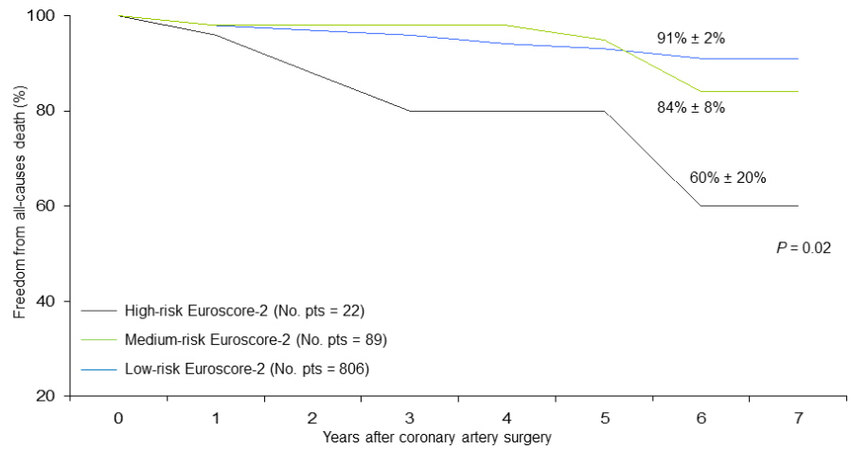
Figure 6. Freedom from late all-cause death in patients undergoing coronary surgery stratified by preoperative EuroSCORE-2 risk profile (low, medium, high).
At the Cox regression analysis, independent predictors of all-cause late death were advanced age at the operation (P < 0.0001), lower preoperative left ventricular ejection fraction (P = 0.01), severe degree of renal dysfunction (P = 0.02), higher Euroscore-2 (P = 0.05), prior PCI (P = 0.03), and more than one stented coronary vessels at the time of prior PCI (P = 0.02) [Table 5].
Independent predictors of late survival (Cox model regression analysis)
| Variables | Hazard ratio | 95%CI | P-value |
| Age at operation (75 vs. 67 years) | 1.12 | 1.08-1.18 | < 0.0001 |
| Left ventricular ejection fraction (0.49 vs. 0.53) | 0.96 | 0.93-0.99 | 0.02 |
| Severe chronic renal dysfunction | 2.50 | 1.11-5.61 | 0.03 |
| Prior PCI vs. primary CABG | 0.06 | 0.01-0.79 | 0.03 |
| No. stented coronary vessels at the time of prior PCI (≥ 2 vessels vs. 1 vessel) | 3.30 | 1.20-8.89 | 0.02 |
| Euroscore-2 (high-risk vs. medium- and low-risk) | 0.29 | 0.08-1.07 | 0.05 |
Prior PCI did not negatively affect late freedom from cardiac death or MACE. The advanced age at the operation was the only independent predictor of late cardiac death (P = 0.02) [Table 6].
Independent predic tors of late cardiac death (Cox model analysis regression)
| Variables | Hazard Ratio | 95%CI | P-value |
| Age at operation (73 vs. 67 years) | 1.1 | 1.01-1.15 | 0.02 |
| Left ventricular ejection fraction (0.48 vs. 0.52) | 0.06 |
DISCUSSION
PCI was thought to be indicated in less extensive coronary artery disease, whereas CABG had shown better results in complex multivessel coronary artery disease. However, in the current clinical practice, PCI is also performed in patients affected by multivessel coronary artery disease with low-medium complexity, in consideration of the five-year results of the SYNTAX trial[11]. Moreover, the SYNTAX trial II showed a substantial improvement in the efficacy of the new-generation drug-eluting stents to reduce MACE (10.6% vs. 17.4%) and the need for repeat revascularization in comparison with SYNTAX I[12]. Nevertheless, CABG remains superior to PCI for complex multivessel disease as well as for overall reduction in the need for repeat revascularization. For these reasons, patients already undergoing PCI are increasingly being subjected to CABG, i.e., 121 patients with prior PCI out of 938 CABG patients (12.9%) in our study.
Early results
Several studies have published worse early, i.e., higher operative mortality, in patients with prior PCI in comparison with primary CABG, with an odds ratio ranging from 1.22 to 9.97 of increased risk[7]. In our analysis, we found that the clinical profile of patients who had previously undergone PCI was worse at the time of CABG surgery in terms of both the severity of ischemic cardiac disease and the associated comorbidity [Table 1]. In our study, in patients with prior PCI, the operative mortality was six times higher in percentage in comparison with those undergoing primary CABG, with a hazard ratio of 4.23. The incidence of postoperative low cardiac output syndrome and renal complications was three times higher
Several possible mechanisms can explain why prior PCI may negatively affect the outcome of CABG surgery. First, prior PCI may limit the number of distal anastomoses which are performed during subsequent CABG. In fact, in patients with an occluded stent, it may be technically difficult to graft the coronary artery distal to a stent if it has been positioned in the middle portion of the coronary vessel, thus leaving areas at risk for ischemia and contractile dysfunction postoperatively. Moreover, vessels with patent stents left non-grafted during CABG might lead to postoperative myocardial infarction if the stent undergoes thrombotic occlusion, given the postoperative pro-thrombotic state and the perioperative cessation of antiplatelet aggregation therapy. Second, prior PCI may reduce the patency of coronary artery bypass grafts. This is because the distal run-off from the graft may be impaired or hindered by multiple overlapping stents compromising collateral blood flow or because the surgeon is forced to graft a more distal segment of the coronary artery due to stent(s) previously placed in the proximal-middle segment. Third, it has become increasingly clear that stents in general, and drug-eluting stents in particular, may affect coronary artery endothelial function[15-18]. Fourth, the PCI procedure can initiate a sequence of inflammatory reactions leading to late post-stenting structural vessel alterations, which make the coronary distal to the stent a poor target vessel for revascularization. Fifth, the anatomical pictures described can worsen in the presence of PCI repeated several times. Massoudy et al.[4] showed that a history of two or more previous PCI was significantly associated with in-hospital mortality (OR 1.9, P = 0.0016) and MACE (OR 1.5, P = 0.0019). Moreover, patients who have PCI and subsequently present for CABG may represent a cohort of patients with more aggressive atherosclerosis[19,20] and, consequently, a more unstable clinical picture. In our population, patients with prior PCI presented with a more unstable clinical picture: two-thirds of patients had unstable angina or recent myocardial infarction, more than 50% had had a previous myocardial infarction, and the mean value of ventricular ejection fraction was < 0.50.
Furthermore, this possibly more compromised coronary anatomic picture in patients with prior PCI in our experience made CABG surgery longer, with superior cardiopulmonary and aortic cross-clamping times, which, although not statistically different from those observed in patients with primary CABG [Table 2], nor identified as operative risk factors in the univariate and multivariate analysis, testified to greater technical complexity.
Finally, it must be emphasized that, although the two groups of patients apparently did not show great preoperative clinical differences and appeared to be well-matched in the analysis, the major explanation for higher mortality in the prior-PCI group is to be attributed to the fact that such patients were overall sicker, and with more advanced heart disease. In fact, as shown in Table 4, the causes of operative death in patients undergoing prior PCI were in 7 out of 8 cases (87.5%) directly related to cardiac problems, while 5 out of 13 cases in patients undergoing primary CABG (38.5%). On the basis of the hospital results analyzed, it could therefore be argued that more CABG interventions should be performed in clinical practice than PCI, but from our study, it is not possible to state this clearly.
All of these aspects may put the patient with prior PCI at higher operative risk and explain the higher observed operative mortality.
Late results
The possible negative effect of prior PCI on medium- and long-term prognosis after CABG is still a matter of open debate[8,21]. In some studies, it is reported that the worst long-term prognosis in terms of reduced survival and freedom from adverse cardiac events may depend on the worst clinical presentation of patients undergoing prior PCI and the anatomical-functional alterations of the coronary arteries site of the stent(s), as already analyzed and discussed regarding the perioperative outcome.
However, in some papers, prior PCI is identified as a predictor of reduced survival, but a more detailed analysis is not reported on the impact that prior PCI may have more specifically on cardiac mortality or freedom from MACE, myocardial infarction, and new revascularization[9,21]. In our analysis, prior PCI was found to be one of the predictors of mortality for any cause in the Cox regression, but not in the survival curve analysis test. In particular, however, prior PCI was identified as a predictor of late cardiac death or MACE ([Tables 5 and 6] and [Figures 2-4]).
On the contrary, other factors contributed more significantly to condition late survival: in fact, in patients affected by preoperative severe renal dysfunction, it was reduced by about 20% (Log-rank test P < 0.0001;
Therefore, our observations appear to be in agreement with other studies that do not demonstrate a worse late outcome of patients undergoing CABG with prior PCI, at least at medium-term follow-up. Indeed, both freedoms from cardiac death (96% vs. 96%, [Figure 3]) and MACE (93% vs. 92%, [Figure 4]) were satisfactory in both patient groups.
The reasons for a similar outcome observed between the two groups of patients are not completely clear, but it could be attributable to some aspects related to surgical revascularization. The territory of LAD was always revascularized, and this factor could undoubtedly improve the long-term prognosis of the studied population. Secondly, for the LAD territory, the left internal thoracic artery provides a long-term benefit and improvement in survival. It has been reported that long-term survival is predominantly conditioned more by a good left internal thoracic artery-LAD graft rather than by an incomplete revascularization of the other non-LAD territories[22-24]. In our series, left internal thoracic artery was used in 91% and 92% of cases [Table 2]. Finally, in both groups of patients, the achieved mean number of grafts per patient was greater than 2.5, i.e., 2.6 vs. 2.8 [Table 2], indicating quite sufficient completeness of revascularization, thus likely providing satisfactory freedom from MACE, including in prior PCI patients[25-28].
Our study has certain limitations. The first limitation is inherent to its retrospective, nonrandomized nature. A study with the propensity score matching of the two groups of patients was not carried out. Moreover, we did not perform a specific analysis to assess the mode of failure of PCI, the intra-stent restenosis vs. de novo development of atherosclerotic disease in the native vessels, and there was a lack of cardiac coronary angiography data at the time of initial PCI to ascertain if PCI was performed in the presence of single- or multivessel coronary disease. Another limitation is dictated by the follow-up length, precisely in the medium term, which does not allow us to draw conclusions on a long-term period of observation. However, we analyzed the patient population of coronary artery bypass surgery from 2015 to 2020, as we have a completer computerized database regarding intraoperative and follow-up data, given that the clinical variables we studied in this work were multiple, and we wanted to get as complete patients data as possible, in order to analyze the available data more precisely.
The specific value of our study may lie in its real-life investigation nature, having included all patients consecutively subjected to coronary surgery over a 6-year period.
In conclusion, patients undergoing CABG after prior PCI have worse perioperative outcomes. Mid-term reduced survival in the prior-PCI patients is mainly due to the concomitant presence of worse clinical presentation and increased comorbidity. Freedom from cardiac death and adverse cardiovascular events are comparable and satisfactory in both cohorts, highlighting the positive protective effect of CABG surgery over time.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study: Nardi P, Ruvolo G
Made contributions to perform data analysis and interpretation: Nardi P
Data acquisition, provided administrative, technical and material support: Asta L, Trombetti D, Bassano C, Bertoldo F, Pisano C, Buioni D, Ferrante MS, Salvati AC, Scognamiglio M
Echocardiographic data acquisition and interpretation: Altieri C
Availability of data and materialsSurgical and Clinical Data Base of Tor Vergata University Polyclinic.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe study was performed in accordance with the Declaration of Helsinki and it was approved by the Institutional Review Board of the Tor Vergata Polyclinic (Registry Number 44/22). All patients gave informed surgical consent.
Consent for publicationNot Applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis 2016;8:E1150-62.
2. Rai P, Taylor R, Bittar MN. Long-term survival in patients who had CABG with or without prior coronary artery stenting. Open Heart 2020;7:e001160.
3. Yap CH, Yan BP, Akowuah E, et al. Does prior percutaneous coronary intervention adversely affect early and mid-term survival after coronary artery surgery? JACC Cardiovasc Interv 2009;2:758-64.
4. Massoudy P, Thielmann M, Lehmann N, et al. Impact of prior percutaneous coronary intervention on the outcome of coronary artery bypass surgery: a multicenter analysis. J Thorac Cardiovasc Surg 2009;137:840-5.
5. Mehta GS, LaPar DJ, Bhamidipati CM, et al. Previous percutaneous coronary intervention increases morbidity after coronary artery bypass grafting. Surgery 2012;152:5-11.
6. Mariscalco G, Rosato S, Serraino GF, et al. Prior percutaneous coronary intervention and mortality in patients undergoing surgical myocardial revascularization: results from the E-CABG (European multicenter study on coronary artery bypass grafting) with a systematic review and meta-analysis. Circ Cardiovasc Interv 2018;11:e005650.
7. Altarabsheh SE, Deo SV, Hang D, et al. Coronary artery bypass grafting after percutaneous intervention has higher early mortality: a meta-analysis. Ann Thorac Surg 2015;99:2046-52.
8. Biancari F, Mariscalco G, Rubino AS, et al. The effect of prior percutaneous coronary intervention on the immediate and late outcome after coronary artery bypass grafting: systematic review and meta-analysis. Heart Lung Vessel 2014;6:244-52.
9. Mannacio V, Di Tommaso L, De Amicis V, et al. Previous percutaneous coronary interventions increase mortality and morbidity after coronary surgery. Ann Thorac Surg 2012;93:1956-62.
10. Nardi P, Pisano C, Bertoldo F, et al. Warm blood cardioplegia versus cold crystalloid cardioplegia for myocardial protection during coronary artery bypass grafting surgery. Cell Death Discov 2018;4:23.
11. Mohr FW, Morice M, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. The Lancet 2013;381:629-38.
12. Escaned J, Collet C, Ryan N, et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J 2017;38:3124-34.
13. Eifert S, Mair H, Boulesteix AL, et al. Mid-term outcomes of patients with PCI prior to CABG in comparison to patients with primary CABG. Vasc Health Risk Manag 2010;6:495-501.
14. Bonaros N, Hennerbichler D, Friedrich G, et al. Increased mortality and perioperative complications in patients with previous elective percutaneous coronary interventions undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2009;137:846-52.
15. Shin DI, Kim PJ, Seung KB, et al. Drug-eluting stent implantation could be associated with long-term coronary endothelial dysfunction. Int Heart J 2007;48:553-67.
16. Muhlestein JB. Endothelial dysfunction associated with drug-eluting stents what, where, when, and how? J Am Coll Cardiol 2008;51:2139-40.
17. Hassan A, Buth KJ, Baskett RJ, et al. The association between prior percutaneous coronary intervention and short-term outcomes after coronary artery bypass grafting. Am Heart J 2005;150:1026-31.
18. Thielmann M, Leyh R, Massoudy P, et al. Prognostic significance of multiple previous percutaneous coronary interventions in patients undergoing elective coronary artery bypass surgery. Circulation 2006;114:I441-7.
19. Stone PH, Coskun AU, Yeghiazarians Y, et al. Prediction of sites of coronary atherosclerosis progression: in vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol 2003;18:458-70.
20. Alfonso F, Hernández C, Pérez-vizcayno MJ, et al. Fate of stent-related side branches after coronary intervention in patients with in-stent restenosis. J Am Coll Cardiol 2000;36:1549-56.
21. Luthra S, Leiva Juárez MM, Senanayake E, et al. Percutaneous intervention before coronary artery bypass surgery does not unfavorably impact survival: a single-center propensity-matched analysis. Ann Thorac Surg 2016;102:1911-8.
22. Rastan AJ, Walther T, Falk V, et al. Does reasonable incomplete surgical revascularization affect early or long-term survival in patients with multivessel coronary artery disease receiving left internal mammary artery bypass to left anterior descending artery? Circulation 2009;120:S70-7.
23. Head SJ, Mack MJ, Holmes DR Jr, et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg 2012;41:535-41.
24. Taggart DP. Incomplete revascularization: appropriate and inappropriate. Eur J Cardiothorac Surg 2012;41:542-3.
25. Miguel GSV, Sousa AG, Silva GS, Colósimo FC, Stolf NAG. Does prior percutaneous coronary intervention influence the outcomes of coronary artery bypass surgery? Braz J Cardiovasc Surg 2020;35:1-8.
26. Cheng YT, Chen SW, Chang CH, et al. Impact of prior coronary stenting on the outcome of subsequent coronary artery bypass grafting. Biomed J 2017;40:178-84.
27. Ghodbane W, Ragmoun W, Arbi R, et al. Correlation between previous coronary artery stenting and early mortality in patients undergoing coronary artery bypass graft surgery. Ann Cardiol Angeiol 2013;62:429-34.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Nardi P, Asta L, Trombetti D, Bassano C, Bertoldo F, Pisano C, Buioni D, Ferrante MS, Salvati AC, Scognamiglio M, Altieri C, Ruvolo G. Early and mid-term results in patients undergoing primary CABG in comparison with patients with PCI prior to CABG. Vessel Plus 2022;6:61. http://dx.doi.org/10.20517/2574-1209.2022.13
AMA Style
Nardi P, Asta L, Trombetti D, Bassano C, Bertoldo F, Pisano C, Buioni D, Ferrante MS, Salvati AC, Scognamiglio M, Altieri C, Ruvolo G. Early and mid-term results in patients undergoing primary CABG in comparison with patients with PCI prior to CABG. Vessel Plus. 2022; 6: 61. http://dx.doi.org/10.20517/2574-1209.2022.13
Chicago/Turabian Style
Nardi, Paolo, Laura Asta, Daniele Trombetti, Carlo Bassano, Fabio Bertoldo, Calogera Pisano, Dario Buioni, Maria Sabrina Ferrante, Alessandro Cristian Salvati, Mattia Scognamiglio, Claudia Altieri, Giovanni Ruvolo. 2022. "Early and mid-term results in patients undergoing primary CABG in comparison with patients with PCI prior to CABG" Vessel Plus. 6: 61. http://dx.doi.org/10.20517/2574-1209.2022.13
ACS Style
Nardi, P.; Asta L.; Trombetti D.; Bassano C.; Bertoldo F.; Pisano C.; Buioni D.; Ferrante MS.; Salvati AC.; Scognamiglio M.; Altieri C.; Ruvolo G. Early and mid-term results in patients undergoing primary CABG in comparison with patients with PCI prior to CABG. Vessel Plus. 2022, 6, 61. http://dx.doi.org/10.20517/2574-1209.2022.13
About This Article
Copyright
Data & Comments
Data
 Cite This Article 8 clicks
Cite This Article 8 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.