Immunomodulatory role of EV-derived non-coding RNA in lung cancer
Abstract
Lung cancer is the deadliest cancer worldwide, primarily because of its metastatic spread. Extracellular vesicles (EVs) are small lipid-bilayer particles released by almost all types of cells. EVs play fundamental roles in cell-cell communication and cell-environment interactions by carrying proteins, nucleic acids such as DNA and RNA (mRNAs, lncRNAs, and miRNAs), and other bioactive molecules that are able to influence the behaviour of recipient cells. EVs have been described as key players in the modulation of tumour progression and the anticancer immune response. In this review, we highlight current knowledge on the role of non-coding RNAs in the modulation of the immune response, focusing on lung cancer. Since EVs are fundamental cell-to-cell mediators, we discuss the current knowledge on the immunomodulatory properties of tumour-derived EVs and, in particular, their ncRNA cargo during the different phases of lung cancer development and progression.
Keywords
LUNG CANCER
Lung cancer is the leading cause of tumour-related death worldwide for both men and women[1]. Generally, this disease is classified as non-small-cell lung cancer (NSCLC, 85%) and small-cell lung cancer (SCLC, 15%)[2]. Histologically, NSCLC is subdivided into squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, which are the most prevalent types[3]. For NSCLC, the 5-year survival rate is estimated to be approximately 15% due to late diagnosis, the presence of tumour heterogeneity, and the limited understanding of lung cancer pathogenesis[4]. For early-stage NSCLC, surgical resection is the best therapeutic option and is applied alone or in combination with platinum-based chemotherapy, whereas chemotherapy and radiation represent the treatment of choice for advanced or metastatic lung cancer patients. The identification of driver mutations and genetic rearrangements in approximately 50%-60% of NSCLC cases has led to a change in the treatment of subgroups of lung cancer patients with a specific molecular profile[5,6]. Recently, improvements were achieved in the management of lung cancer as a result of the development of immune checkpoint inhibitors (ICIs) that block the PD-L1/PD1 axis or CTLA-4[7]. Currently, immunotherapy alone or in combination with standard chemotherapy represents a more promising therapeutic option for advanced-stage lung cancer than standard chemotherapy[8]. However, no reliable biomarkers are available to stratify patients who will benefit from this therapeutic approach, emphasizing the need to better understand the molecular processes underlying lung cancer development.
The cancer microenvironment has important impacts on the development and progression of lung tumours[9]. The tumour microenvironment (TME) includes endothelial cells, cancer-associated fibroblasts, and infiltrating immune cells[10,11]. Tumour cells are able to modulate the surrounding environment through the release of several elements, such as cytokines and extracellular vesicles (EVs)[12]. EVs can act as mediators of cellular communication through the delivery of their cargoes, such as proteins, lipids, and non-coding RNAs (ncRNAs)[13,14]. In this review, we first discuss the role of ncRNAs in the modulation of the immune response in the lung cancer microenvironment and then describe how EVs released from cancer cells modulate the phenotype of infiltrating immune cells to support tumour growth or eliminate tumour cells. Finally, we focus on the importance of ncRNAs carried by EVs from lung cancer cells and their immunoregulatory activity.
Immune system and lung cancer
Currently, there is a consensus about the importance and clinical relevance of the immune system and cancer interactions during all phases of tumour progression[15]. Indeed, the acquisition of oncogenic mutations by non-malignant cells is not sufficient for the full transition to a malignant phenotype. In this regard, several other modifications within the microenvironment are required to fuel cancer cells with nutrients, impair cell death pathways, and, most importantly, help mutant cells escape the control of the immune system[16]. Indeed, both the innate and adaptive immune systems can recognize and eliminate cancer cells[17]. Normally, the innate immune system, composed of natural killer (NK) cells, polymorphonuclear (PMN) leukocytes, mast cells, and antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs), is faster than the adaptive immune system in recognizing and eliminating cancer cells through the production of inflammatory cytokines, including interferon-gamma (IFN-γ), and perforin[18]. Conversely, adaptive immunity (mainly mediated by T and B cells) takes longer to initiate a response, but it is active after the recognition of specific antigens displayed on the surface of cancer cells, which elicits a more robust and durable anticancer response. However, during cancer progression, cancer cells acquire the capability to avoid immune recognition by adopting different immune escape mechanisms, such as defective processing and MHC class I presentation of cancer-related antigens and the creation of an immunosuppressive microenvironment[19]. The latter condition is established through the recruitment of suppressive immune cells, the polarization of immune and stromal cells towards a pro-tumoral phenotype, the production of immunosuppressive cytokines, or the tumour or stromal cell expression of inhibitory immune checkpoint molecules (e.g., CTLA-4 and PD-L1) that can negatively affect the proper functioning of tumour-infiltrating lymphocytes. Together, these alterations strongly impair the immune system, which becomes unable to recognize and eliminate tumour cells, resulting in tumour progression and outgrowth[20].
In the context of the lung cancer microenvironment, two important studies investigated tumour-induced infiltrating lymphoid and myeloid cells and their reprogramming capacity[21,22]. Lavin et al. described the first innate immune cell atlas of early lung adenocarcinoma lesions, reporting the impaired balance between infiltrating effector CD8+ T cells and T regulatory cells (Tregs) at the tumour site, observed as a decline in T cells expressing granzyme B and IFN-γ coupled with an expansion of suppressor T cells[21]. While studying the innate immunity compartment, Durrans et al. noticed an increased number of bone marrow-derived cells in tumour samples compared to corresponding normal tissue samples[22]. In detail, increased production of pro-tumoral factors, mainly osteopontin and the chemokine CCL7, was detected within the tumour microenvironment (TME) and attributed specifically to myeloid cells (both immature monocytic myeloid cells and neutrophils)[22].
Similarly, Lavin et al. described several alterations within the TME: the paucity and dysfunction of NK cells, dendritic cells (DCs), and CD16+ monocytes, along with an increase in immunosuppressive macrophages[21]. In addition, single-cell RNA sequencing revealed that macrophages present within a tumour, which were mainly derived from monocytes with immunosuppressive activity, showed a significantly different transcriptional profile than normal tissue macrophages[23]. Interestingly, data from early lung adenocarcinoma showed that tumour-associated macrophages (TAMs) expressed the immunomodulatory transcription factor PPARγ, CD64, CD14, and CD11c and had reduced expression of CD86 and CD206[21].
ncRNAs and immune regulation in lung cancer
For a long time, proteins were believed to be the only products derived from genetic information having functional significance. For this reason, studying the specific regions of the genome that encode proteins is an appealing field of interest in medical research. Innovative sequencing tools, however, have revealed that the protein-coding region accounts for only 2% of the whole genome and that the remaining 98% encodes thousands of RNA molecules with essential biological and pathological roles as process regulators[24]. Historically, these RNAs, known as ncRNAs, were classified into two main categories based on their size: small ncRNAs and long ncRNAs (lncRNAs). Small RNAs are less than 50 nucleotides in length and include microRNAs (miRNAs), ribosomal RNA (rRNA), transfer RNA (tRNA), and piwi-interacting RNA (piRNA). On the other hand, lncRNA segments contain longer sequences, generally exceeding 200 nucleotides, and include pseudogenes and circular RNAs (circRNAs)[25]. Among small ncRNAs, miRNAs are the most studied and described in cancer progression[26]. MiRNAs are a family of non-coding RNAs composed of 21-25 nucleotides, and their biogenesis is a multistep process that involves the processing of RNA transcripts. MiRNAs are involved in a huge number of functions varying from the transcriptional/post-transcriptional level to the translational level, meaning that miRNAs can regulate a great number of messenger RNAs in a cell[27]. It has been proven that a single miRNA can usually modulate several genes and that one gene can be controlled by multiple miRNAs[28]. Indeed, this family of ncRNAs is implicated in the regulation of several gene networks through the modulation of oncogenes such as RAS, MYC, and EGFR and tumour suppressors such as TP53, PTEN, and BRCA1[29].
NcRNAs have also been implicated in the regulation of immune cell signalling in lung cancer. The most relevant works describing how ncRNAs modulate immune cell recruitment and functions are summarized in Table 1. A study on NSCLC detected PD-L1 as a downstream target of miR-200/ZEB1, and this targeting contributed to immunosuppression in primary tumour tissue by increasing T-cell exhaustion[30]. Moreover, another work by Fujita et al. demonstrated the correlation between miR-197 expression and the down-regulation of CKS1B, a key regulator of PD-L1 synthesis[31]. On the other hand, miR-3127 was shown to promote PD-L1 overexpression and immune escape in lung adenocarcinoma through STAT3 phosphorylation[32].
Role of ncRNAs in the regulation of immune response in lung cancer
| References | ncRNA | Target | Function |
| Chen et al.[30] | miR-200 | ZEB1 and PD-L1 | T cell exhaustion |
| Fujita et al.[31] | miR-197 | Cyclin-dependent kinase subunit 1 (CKS1B) | Induce tumour progression |
| Tang et al.[32] | miR-3127 | STAT3/PD-L1 | Sustain immune escape |
| Sun et al.[34] | lnCRNA XIST | IL-10 and CD163 down-modulation | Conversion to M2-like macrophages |
| Li et al.[35] | GNAS-AS1 | mir-4319 | Promote NSCLC cell growth and metastasis |
| Tian et al.[36] | lncRNA HOTAIRM1 | HOXA | Increase CD8+ cytotoxic T lymphocyte cells |
| Wu et al.[37] | circ_0020714 | miR-30a-5p/SOX4 | Immune evasion and anti-PD-1 resistance |
| Yang et al.[38] | CHST1 | miR-155 and miR-194 | Promote immune escape of lung cancer |
Along with miRNAs, lncRNAs have been shown to play a role in the anti-tumour immune response in lung cancer. In a recent article, Sage et al. combined single-cell RNA-sequencing data and flow-sorted healthy peripheral blood mononuclear cell (PBMC) data to identify immune-related lncRNAs with the potential to identify infiltrated immune cell populations within tumours[33]. Furthermore, considering the role of lncRNAs in the regulation of the expression of oncogenic genes, this information could be correlated with the deregulation of several gene pathways in cancer[33]. Sun et al. reported that lung cancer cells induced the up-regulation of the lncRNA XIST on macrophages and that this mechanism promoted conversion to an M2-like macrophage phenotype[34]. Furthermore, this conversion was characterized by the down-regulation of specific markers such as IL-10 and CD163, which subsequently promoted invasion and migration by lung cancer cells. In this study, the authors proved that the conditioned medium of lung cancer cells induced XIST and promoted the expression of M2-related genes in macrophages[34].
LncRNAs such as GNAS-AS1 were found to regulate the expression of mir-4319 in in vitro-differentiated THP-1 macrophages, thus increasing their number and consequently promoting NSCLC cell growth and metastasis[35]. In contrast, overexpression of the lncRNA HOTAIRM1 can reduce the immunosuppressive properties of MDSCs. In particular, this lncRNA, through the up-regulation of its target HOXA1, negatively affects the production of immunosuppressive molecules by MDSCs, thus reducing the immune suppression mediated by these pro-tumoral cells[36].
It has been demonstrated that many types of circular RNAs are involved in NSCLC immune evasion. Indeed, circ_0020714 was found to be up-regulated in NSCLC tissues compared with non-tumour adjacent tissues, where it acted as a sponge for mir-30a-5p, which in turn up-regulated the levels of the transcription factor SOX4[37]. Furthermore, the circRNA CHST15 has been described to act as an oncogene in lung cancer since its down-modulation correlates with reduced tumour growth. Moreover, CHST15, by sponging miR-155 and miR-194, promotes the expression of PD-L1 on tumoral cells, thus contributing to immune escape during tumour progression[38].
EXTRACELLULAR VESICLES
“Extracellular vesicles” (EVs) are a generic term describing the lipid-bilayer particles released by almost all cells in the human body. However, these particles are highly heterogeneous, and their classification can differ based on the criteria utilized to differentiate them. EVs originate mainly via two cellular routes, one involving the endocytic cellular pathway and the other involving the plasma membrane. Exosomes, or small EVs, are endosome-derived particles with a mean size of 50-150 nm. Their release relies on the formation of multivesicular bodies in the endosomal compartment and their subsequent fusion with the plasma membrane. In contrast, microvesicles, or ectosomes, have a larger mean size than exosomes (100-1,000 nm), and their formation depends on outwards budding from the plasma membrane[39]. Although this classification has been widely used, the cellular origin of these particles remains a challenging issue, as there is no consensus within the EV community on the markers utilized to discriminate different EVs subtypes based on their origin[40]. For these reasons, the guidelines of the International Society for Extracellular Vesicles (ISEV) suggest the use of “extracellular vesicles” to indicate isolated particles and the adoption of a classification system based on the physical characteristics of EVs, such as dimension, density, and biochemical properties[40].
Due to their intrinsic properties, EVs act as cellular messengers by carrying different bioactive molecules (proteins, nucleic acids, and metabolites) from one cell to another recipient cell, suggesting their pivotal role in cell-to-cell communication. EVs-related molecules can participate in many biological processes in different pathological conditions[41]. In cancer, EVs have been described as mediators in tumour progression-related mechanisms through the modulation of vascular permeability and neoangiogenesis, which allows and supports cell extravasation and metastatic outgrowth. EVs have also been reported to be involved in the modulation of the anticancer immune response[42,43].
Immunomodulatory functions of tumour-derived EVs
As described in the previous section, an important process in tumour progression is tumour escape: a state where tumour cells, via different mechanisms, prevent their recognition and consequent elimination by immune cells, resulting in tumour growth[19]. Among the different signals that can hamper the proper functioning of immune cells are interactions with tumour-derived EVs (tEVs)[44,45]. In particular, tEVs can have opposite effects: while they can suppress immune cell function, they can also express different tumour antigens with immunogenic properties on their surface[46] [Table 2].
Immunomodulatory properties of tumour derived-EVs
| References | EVs origin | Specimens | Isolation method | tEVs effective molecule | Functional role |
| Pro-tumoral immune response | |||||
| Chen et al.[48] | Melanoma | cell lines | Ultracentrifugation | PD-L1 | Inhibition of T cells proliferation and functionality |
| Ohue et al.[50] | Melanoma | cell lines | Capture beads | Induction of TLR3-TRIF signaling in DCs with IFN-β production | Increased number of tumour-infiltrating Treg and tumour outgrowth |
| Nakazawa et al.[51] | Leukemia | cell lines and plasma | Ultracentrifugation for cell lines and SEC for plasma samples | 4-1BBL/CD137L molecules | Activation of Treg cells |
| Haderk et al.[53] | B-chronic lymphocytic leukaemia | cell line | Ultracentrifugation and sucrose density cushion | Non-coding Y RNA hY4 | Induction of PD-L1 expression on monocytes |
| Vignard et al.[54] | Melanoma | cell lines | Ultracentrifugation and ExoQuick® | miR-3187-3p, miR-498, miR-122, miR-149, and miR-181a/b | Reduced TCR signaling pathway and cytotoxic activity in CD8+ T cells |
| Shinohara et al.[55] | Colorectal cancer | cell lines | Ultracentrifugation | miR-145 | M2-like polarization via histone deacetylase 11 down modulation |
| Xun et al.[56] | Breast carcinoma | cell lines | Ultracentrifugation | miRNA-138-5p | M2-like polarization via H3K27 histone demethylase KDM6B inhibition |
| Zhang et al.[57] | Glioblastoma and microglia | cell lines | Ultracentrifugation | circular RNA circ_0012381, miR-340-5p | Increased secretion of CCL2 by microglia cells that in turn promotes tumour growth |
| Antitumoral immune response | |||||
| Menay et al.[58] | T lymphoma | mouse model | Sucrose density cushion | CD24 and Hsp90 | Generation of specific humoral and cellular immune response |
| Daßler-Plenker | Melanoma | cell lines | Ultracentrifugation | NKp-30 ligands (BAG6, BAT3) | Activation of the cytotoxic activity of NK cells via NKp-30 receptor |
| Ma et al.[60] | Melanoma | cell lines | Ultracentrifugation | TAAs | Promotion of MHC class I: TAAs complex formation in DC |
A well-described process through which tEVs inhibit the functions of immune cells is the expression of immunoregulatory molecules, such as PD-L1, on their surface[47]. PD-L1 on melanoma-derived EVs was observed to inhibit the proliferation and cytotoxic activity of CD8+ T cells in vitro[48]. The suppression mediated by PD-L1-EVs required an interaction between ICAM-1, which is up-regulated together with PD-L1 following IFN-γ stimulation, and LFA-1, which is expressed on activated T cells. Indeed, the blockade of ICAM-1 on EVs prevented the interaction of melanoma-EVs with CD8+ T cells and the consequent inhibition mediated by PD-L1[49]. Notably, the immunosuppressive properties of EVs expressing PD-L1 were demonstrated in models involving EVs from tumour cell lines, which could differ from patient-derived EVs and their real anti-immune activity.
CD4+ Tregs represent important helper cells involved in tumour growth since they down-regulate the cytotoxic antitumour activity of CD8+ T cells, and their presence within a tumour correlates with a poor prognosis in different cancer types[50]. In melanoma-bearing mice, the internalization of tEVs by DCs was shown to stimulate IFN-β production via the endosomal TLR3 signalling pathway, resulting in an increased number of Tregs and tumour outgrowth[51]. Leukaemia-derived EVs (obtained from either human cell lines or patient plasma) that transport 4-1BBL/CD137L molecules were shown to induce activation and effector phenotypes in Tregs via the upregulation of CD39 and TNFR2 expression[52].
Impairment of immune function can be achieved by other mechanisms that involve several types of non-coding RNA. In chronic lymphocytic leukaemia, the non-coding Y RNA hY4 enriched in tumour exosomes induces the expression of PD-L1 on monocytes via stimulation of endosomal TLR7 signalling, thus promoting tumour escape[53]. In contrast, the TCR and TNF-α signalling pathways in CD8+ T cells are disrupted by the activity of miR-3187-3p, miR-498, miR-122, miR-149, and miR-181a/b delivered by melanoma-derived EVs[54]. In addition, non-coding RNAs associated with tEVs also influence the polarization of tumour-infiltrated macrophages towards an M2 phenotype, which is fundamental for tumour progression. For instance, in colorectal cancer, this polarization occurs via the down-modulation of histone deacetylase 11 mediated by miR-145 within colorectal cancer cell-derived EVs[55]. In addition, miR-138-5p, which has been observed in breast cancer-derived EVs, induces an M2-like phenotype in macrophages and promotes tumour growth by inhibiting the H3K27 histone demethylase KDM6B[56]. On the other hand, in brain malignancies, the uptake by microglia of EV-sorted Circular RNAs (circRNA) circ_0012381, which sponges with miR-340-5p, increases ARG1 expression, resulting in CCL2 secretion, which in turn promotes the growth of glioblastoma cells[57].
On the one hand, tEVs are able to affect immune function negatively, as largely discussed above; on the other hand, the same tEVs can elicit a response against tumours by stimulating different immune populations. Indeed, tEVs obtained from the ascites of T-cell lymphoma-bearing mice expressed CD24 and Hsp90, malignant markers, on their surface and induced a competent immune response resulting in the rejection of a subsequent tumour challenge in syngeneic naïve mice[58]. Daßler-Plenker et al. showed that stimulation of the cytosolic immune sensor RIG-I in melanoma cells affected the protein surface expression of tEVs, promoting NK cell functionality[59]. tEVs can also positively affect the presentation of tumour associated antigens (TAAs) by DCs and thus have an intrinsic potential as a vaccine. For instance, the endocytosis of melanoma-derived microparticles efficiently promoted the formation of MHC class I-tumour antigen complex together with the induction of the costimulatory molecules CD80 and CD86. The concomitant expression of these molecules with MHC complexes allowed a highly efficient tumour antigens presentation to CD8+ T cells[60].
EVs released by infiltrating Treg cells could prevent the proper functioning of other T-cell subtypes. Additionally, let-7d in exosomes is transferred to Th1 cells, contributing to immune cell suppression, which demonstrates that within the tumour microenvironment, tumour cells secrete EVs to influence the behaviour of immune cells[61].
Immune cell regulation by non-coding RNA in lung cancer-derived EVs
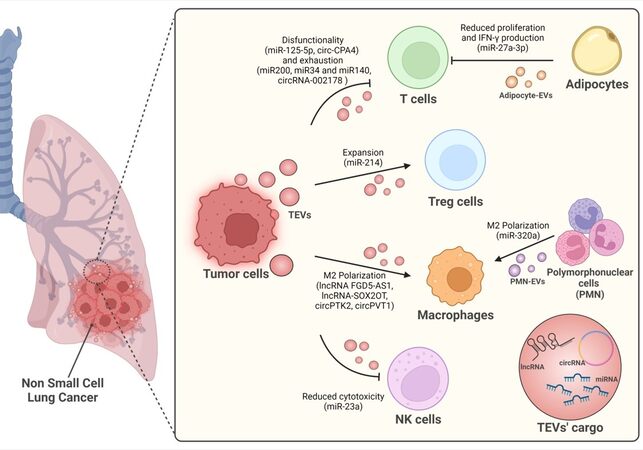
The EV field has widely expanded in recent years, but only a few studies have aimed to understand the role of EVs in immune regulation in the context of lung cancer. Hereafter, we report all the articles showing a link between EV-contained non-coding RNAs and immune regulation in lung cancer. Figure 1 highlights the key roles played by tEVs in affecting the behaviours of different cell populations within the TME and the many different ncRNAs involved in these processes.
MicroRNAs
Hypoxia is one of the most important drivers of lung cancer progression. Indeed, the hypoxic tumour microenvironment strongly affects the release of tEVs[62]. Furthermore, hypoxic tEVs enriched in TGF-β and miR-23a impair NK cell cytotoxic abilities by down-regulating two fundamental receptors of NK cell activation and degranulation (NKG2D and CD107a)[63].
Interestingly, the level of adipocyte-derived miR-27a-3p was observed to decrease as body mass index (BMI) increased, and this was inversely correlated with the level of the costimulatory gene ICOS, which is important in T-cell activation[64]. Although the link between miR-27a-3p and the ICOS gene was not directly demonstrated, in vitro experiments showed that EVs from adipocytes silenced for miR-27a-3p displayed higher levels of ICOS+ T cells and higher levels of IFN‐γ production. Similarly, Peng et al. observed a correlation between the up-regulation of EV-miR-125b-5p and T-cell dysfunction at baseline in nonresponsive NSCLC patients undergoing ICI therapy[65]. Moreover, they identified three miRNAs from the miR-320 family (miR-320d, miR-320c, and miR-320b) associated with a poor prognosis and response to ICIs, identifying these miRNAs as potential biomarkers for therapy response[65]. In addition, our group described circulating miR-320a shuttled by PMN-derived EVs in high-risk heavy smokers, defining its critical role in the induction of a pro-tumorigenic M2-like phenotype in macrophages via STAT4 targeting[66].
An interesting study on T-cell modulation highlighted the role of the miR-200/ZEB1 axis in the modulation of the levels of PD-L1 on lung tumour cells and, consequently, in T-cell exhaustion[30]. A similar role was also attributed to miR-34 and miR-140, which both directly bind PD-L1 in NSCLC cells[67,68]. Furthermore, Treg cell expansion could also be mediated via the regulation of PTEN by miR-214 carried within microvesicles released by different types of cancer, highlighting a possible common mechanism to induce tumour progression[69].
Long non-coding RNAs
Similar to the members of the ncRNA family, lncRNAs have been observed to be deregulated in all stages of lung cancer development[70]. In 2016, Wang et al. first reported the involvement of EV-related lncRNAs in lung cancer by highlighting a new mechanism of interaction between lung tumour cells and their microenvironment[71]. Indeed, EVs produced by lung tumour cells were found to be responsible for a deep alteration in the lncRNA profile in mesenchymal cells. Even if a direct association with lncRNAs in tEVs was not provided, this study described for the first time the role of microenvironmental lncRNA perturbation in lung cancer[71].
Within the NSCLC microenvironment, TAMs are one of the main cellular components: they directly support cancer cell growth, survival, invasion, and metastasis and additionally provide protection to NSCLC cells via immune evasion strategies[72]. Recently, evidence suggesting EV-mediated crosstalk between lung tumour cells and macrophages was reported[73]. Indeed, in lung cancer, the lncRNA FGD5-AS1 detected in tEVs was found to be responsible for phenotypic alterations in macrophages, which resulted in the upregulation of genes involved in M2 polarization[74]. Interestingly, tEV-lncRNA-SOX2OT was detected in the blood of NSCLC patients and linked to the formation of pro-metastatic features by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts[75]. Indeed, SOX2OT was detected inside EVs from NSCLC cells and associated with the induction of an M2-like phenotype and concomitant M1 polarization inhibition through the miR-627-3p/SMAD signalling pathway, resulting in increased EGFR-TKI resistance.
Circular RNAs
Circular RNAs (CircRNAs) are an emerging field in cancer research, especially in NSCLC, as they were demonstrated to play pivotal roles in carcinogenesis, tumour formation, proliferation, migration, invasion, and sensitivity to therapy[76]. The first evidence of the presence of circRNA in cancer EVs was reported in 2015 when Li et al., using RNA-seq methods, demonstrated the enrichment of circRNAs in tEVs compared to the cell of origin[77]. However, although many efforts have been made in recent years to understand the role of circRNAs in cancer progression, their impact on NSCLC has not been investigated as carefully as that of other types of non-coding RNAs. Most studies on EV-associated circRNAs in lung cancer aimed to comprehend their role in tumour cells better; thus, their involvement in the modulation of the immune landscape is still unknown[78].
Interestingly, a multifaceted role for the circ-CPA4/let-7 miRNA/PD-L1 axis in NSCLC was described by Hong et al., showing how circ-CPA4 promoted the production of tumoral-PD-L1+-EVs, which interacted with T cells to establish CD8+ T-cell inactivation, tumour immune escape and resistance to chemotherapy[79]. Similar results were obtained by Wang et al., who demonstrated the presence of high levels of circRNA-002178 in tumour samples and lung cancer cell lines and showed that enhancing PD-L1 expression led to T-cell exhaustion[80]. Importantly, the authors showed that circRNA-002178 was also present in the plasma-EVs of NSCLC patients and that its delivery into CD8+ T cells induced PD1 expression. Regarding the interplay between circRNA-EVs and innate immunity in lung cancer, only a few studies have described the involvement of circRNA-EVs in modulating macrophage polarization. Interestingly, circPTK2 was observed to be highly expressed in lung cancer patient serum EVs and correlated with the cancer stage. Most importantly, macrophages enriched in circPTK2 were found to be relatively pro-tumoral (M2 polarization), suggesting a possible role for circPTK2 in the EV-mediated crosstalk between cancer cells and the stroma[81]. Another circRNA linked to macrophage polarization is circPVT1, which was observed in EVs from lung cancer patients (blood) and cell lines. Indeed, the delivery of circPVT1 to macrophages via EVs was shown to cause M2-like polarization by sponging miR-124 and consequently increasing EZH2 expression. Moreover, the authors showed that co-incubation with EV-treated macrophages prompted lung cancer cell proliferation, migration, and invasion[82]. Taken together, these studies suggest a potential role for EV-circRNA in the modulation of the immune microenvironment. There is still much work to be done to better elucidate the involvement of circRNA-EVs and immune modulation in lung cancer.
CONCLUSION
Extracellular vesicles as modulators of the immune response are still an expanding area of research. Here, we described several studies showing significant roles for these particles as diagnostic or prognostic biomarkers in cancer. However, to reach clinical implementation, several challenges still need to be addressed.
A consensus still needs to be reached among researchers regarding the term “extracellular vesicles” and their utilization, although the ISEV stated its agreement for the use of the term when indicating lipid-bilayer particles released by cells. The inappropriate use of “exosomes” and “microvesicles” creates confusion and misunderstanding among readers[40]. Another issue to address is EV characterization: the majority of the studies investigating the role of EVs in cancers are poorly characterized, as illustrated by the minimal information for studies of extracellular vesicles (MISEV) guidelines[40]. Lack of adherence to these guidelines affects the quality of published findings and the reproducibility of results.
Notably, the immunoregulatory function of tumour-derived EVs has mostly been demonstrated using EVs separated from the conditioned media of tumour cell lines. This approach is completely different from using circulating patient-derived EVs obtained from blood, which are mainly derived from other types of cells. Indeed, all results should be confirmed using cancer patient samples, in which tEVs are present along with EVs of different cellular origins. This would allow us to comprehend whether the role of EVs is strictly correlated to the tumour microenvironment or at the systemic level and, therefore, relevant to immune regulation.
In the last twenty years, non-coding RNAs have emerged as reliable candidates for predictive and prognostic biomarkers and therapeutic targets in cancer. However, implementation of these small molecules in the clinical setting has yet to be ready due to several methodological issues that need to be addressed. In this regard, standardized procedures for ncRNA isolation and detection should be established among researchers to avoid inconsistencies and lack of reproducibility among different studies. Nonetheless, a better comprehension of the origin and mechanisms of release of these molecules is necessary before they are implemented in the clinical setting. Despite these challenges, EVs and their non-coding RNA cargo could represent an interesting tool for cancer treatment management.
To date, research has unveiled pivotal functional EV-related ncRNAs involved in modulating the tumour-immune relationship and suggested their potential value involvement in monitoring and predicting treatment responses in lung cancer patients.
DECLARATIONS
Author’s contributionsWriting-original draft preparation: Ghidotti P, Petraroia I, Fortunato O, Pontis F
Funding acquisition: Fortunato O
All authors have read and agreed to the published version of the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipThe study was supported by a grant from the Italian Ministry of Health (GR-2019-12369047 to
All authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
2. Goldstraw P, Chansky K, Crowley J, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51.
3. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019;94:1623-40.
4. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311.
5. Tartarone A, Lapadula V, Di Micco C, et al. Beyond conventional: the new horizon of targeted therapy for the treatment of advanced non small cell lung cancer. Front Oncol 2021;11:632256.
6. Takeda M, Nakagawa K. First- and second-generation
7. Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol 2021;18:625-44.
8. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 2021;39:2339-49.
9. Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 2019;19:9-31.
10. Barbazán J, Matic Vignjevic D. Cancer associated fibroblasts: Is the force the path to the dark side? Curr Opin Cell Biol 2019;56:71-9.
11. Kargl J, Busch SE, Yang GH, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 2017;8:14381.
12. Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol 2018;6:18.
13. Wang M, Yu F, Ding H, Wang Y, Li P, Wang K. Emerging function and clinical values of exosomal MicroRNAs in cancer. Mol Ther Nucleic Acids 2019;16:791-804.
14. Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther 2020;5:145.
16. Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249-53.
17. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-71.
18. Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother 2012;35:299-308.
19. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565-70.
20. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64.
21. Lavin Y, Kobayashi S, Leader A, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 2017;169:750-765.e17.
22. Durrans A, Gao D, Gupta R, et al. Identification of reprogrammed myeloid cell transcriptomes in NSCLC. PLoS One 2015;10:e0129123.
23. Kim N, Kim HK, Lee K, et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat Commun 2020;11:2285.
24. Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer 2018;18:5-18.
26. Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ. The roles of microRNA in lung cancer. Int J Mol Sci 2019;20:1611.
27. Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 2014;13:622-38.
28. Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther 2013;93:98-104.
30. Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014;5:5241.
31. Fujita Y, Yagishita S, Hagiwara K, et al. The clinical relevance of the miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant non-small-cell lung cancer. Mol Ther 2015;23:717-27.
32. Tang D, Zhao D, Wu Y, et al. The miR-3127-5p/p-STAT3 axis up-regulates PD-L1 inducing chemoresistance in non-small-cell lung cancer. J Cell Mol Med 2018;22:3847-56.
33. Sage AP, Ng KW, Marshall EA, et al. Assessment of long non-coding RNA expression reveals novel mediators of the lung tumour immune response. Sci Rep 2020;10:16945.
34. Sun Y, Xu J. TCF-4 regulated lncRNA-XIST promotes M2 polarization of macrophages and is associated with lung cancer. Onco Targets Ther 2019;12:8055-62.
35. Li Z, Feng C, Guo J, Hu X, Xie D. GNAS-AS1/miR-4319/NECAB3 axis promotes migration and invasion of non-small cell lung cancer cells by altering macrophage polarization. Funct Integr Genomics 2020;20:17-28.
36. Tian X, Ma J, Wang T, et al. Corrigendum: long non-coding RNA HOXA transcript antisense RNA myeloid-specific 1-HOXA1 axis downregulates the immunosuppressive activity of myeloid-derived suppressor cells in lung cancer. Front Immunol 2019;10:2929.
37. Wu J, Zhu MX, Li KS, Peng L, Zhang PF. Circular RNA drives resistance to anti-PD-1 immunotherapy by regulating the miR-30a-5p/SOX4 axis in non-small cell lung cancer. Cancer Drug Resist 2022;5:261-70.
38. Yang J, Jia Y, Wang B, et al. Circular RNA CHST15 sponges miR-155-5p and miR-194-5p to promote the immune escape of lung cancer cells mediated by PD-L1. Front Oncol 2021;11:595609.
39. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019;21:9-17.
40. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750.
41. Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol 2022;23:369-82.
42. Peinado H, Zhang H, Matei IR, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 2017;17:302-17.
43. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 2016;30:836-48.
44. Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS, Khan MK. Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel) 2018;6:69.
45. Zhang L, Sun M, He Z, Sun J, Li H, Luo Q. Multi-functional extracellular vesicles: potentials in cancer immunotherapy. Cancer Lett 2022;551:215934.
46. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol 2021;22:560-70.
47. Serratì S, Guida M, Di Fonte R, et al. Circulating extracellular vesicles expressing PD1 and PD-L1 predict response and mediate resistance to checkpoint inhibitors immunotherapy in metastatic melanoma. Mol Cancer 2022;21:20.
48. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382-6.
49. Zhang W, Zhong W, Wang B, et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev Cell 2022;57:329-343.e7.
50. Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci 2019;110:2080-9.
51. Nakazawa Y, Nishiyama N, Koizumi H, Kanemaru K, Nakahashi-Oda C, Shibuya A. Tumor-derived extracellular vesicles regulate tumor-infiltrating regulatory T cells via the inhibitory immunoreceptor CD300a. Elife 2021:10.
52. Swatler J, Turos-Korgul L, Brewinska-Olchowik M, et al. 4-1BBL-containing leukemic extracellular vesicles promote immunosuppressive effector regulatory T cells. Blood Adv 2022;6:1879-94.
53. Haderk F, Schulz R, Iskar M, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol 2017;2:eaah5509.
54. Vignard V, Labbé M, Marec N, et al. MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunol Res 2020;8:255-67.
55. Shinohara H, Kuranaga Y, Kumazaki M, et al. Regulated polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J Immunol 2017;199:1505-15.
56. Xun J, Du L, Gao R, et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics 2021;11:6847-59.
57. Zhang C, Zhou Y, Gao Y, et al. Radiated glioblastoma cell-derived exosomal circ_0012381 induce M2 polarization of microglia to promote the growth of glioblastoma by CCL2/CCR2 axis. J Transl Med 2022;20:388.
58. Menay F, Herschlik L, De Toro J, et al. Exosomes isolated from ascites of T-Cell lymphoma-bearing mice expressing surface CD24 and HSP-90 induce a tumor-specific immune response. Front Immunol 2017;8:286.
59. Daßler-Plenker J, Reiners KS, van den Boorn JG, et al. RIG-I activation induces the release of extracellular vesicles with antitumor activity. Oncoimmunology 2016;5:e1219827.
60. Ma J, Wei K, Zhang H, et al. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8+ T cells. Cancer Immunol Res 2018;6:1057-68.
61. Okoye IS, Coomes SM, Pelly VS, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014;41:89-103.
62. He G, Peng X, Wei S, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer 2022;21:19.
63. Berchem G, Noman MZ, Bosseler M, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 2016;5:e1062968.
64. Fan X, Wang J, Qin T, et al. Exosome miR-27a-3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac Cancer 2020;11:1453-64.
65. Peng XX, Yu R, Wu X, et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in
66. Pontis F, Roz L, Mensah M, et al. Circulating extracellular vesicles from individuals at high-risk of lung cancer induce pro-tumorigenic conversion of stromal cells through transfer of miR-126 and miR-320. J Exp Clin Cancer Res 2021;40:237.
67. Cortez MA, Ivan C, Valdecanas D, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst 2016;108:djv303.
68. Xie WB, Liang LH, Wu KG, et al. MiR-140 expression regulates cell proliferation and targets PD-L1 in NSCLC. Cell Physiol Biochem 2018;46:654-63.
69. Yin Y, Cai X, Chen X, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res 2014;24:1164-80.
71. Wang S, Li X, Zhu R, Han Q, Zhao RC. Lung cancer exosomes initiate global long non-coding RNA changes in mesenchymal stem cells. Int J Oncol 2016;48:681-9.
72. Conway EM, Pikor LA, Kung SH, et al. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med 2016;193:116-30.
73. Chen J, Sun W, Zhang H, et al. Macrophages reprogrammed by lung cancer microparticles promote tumor development via release of IL-1β. Cell Mol Immunol 2020;17:1233-44.
74. Lv J, Li Q, Ma R, et al. Long Noncoding RNA FGD5-AS1 knockdown decrease viability, migration, and invasion of non-small cell lung cancer (NSCLC) cells by regulating the microRNA-944/MACC1 axis. Technol Cancer Res Treat 2021;20:1533033821990090.
75. Ni J, Zhang X, Li J, et al. Correction: tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis 2021;12:1131.
77. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 2015;25:981-4.
78. Hussen BM, Abdullah SR, Hama Faraj GS, et al. Exosomal circular RNA: a signature for lung cancer progression. Cancer Cell Int 2022;22:378.
79. Hong W, Xue M, Jiang J, Zhang Y, Gao X. Circular RNA circ-CPA4/ let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J Exp Clin Cancer Res 2020;39:149.
80. Wang J, Zhao X, Wang Y, et al. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis 2020;11:32.
81. Katopodi T, Petanidis S, Domvri K, et al. Kras-driven intratumoral heterogeneity triggers infiltration of M2 polarized macrophages via the circHIPK3/PTK2 immunosuppressive circuit. Sci Rep 2021;11:15455.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ghidotti P, Petraroia I, Fortunato O, Pontis F. Immunomodulatory role of EV-derived non-coding RNA in lung cancer. Extracell Vesicles Circ Nucleic Acids 2023;4:59-71. http://dx.doi.org/10.20517/evcna.2022.42
AMA Style
Ghidotti P, Petraroia I, Fortunato O, Pontis F. Immunomodulatory role of EV-derived non-coding RNA in lung cancer. Extracellular Vesicles and Circulating Nucleic Acids. 2023; 4(1): 59-71. http://dx.doi.org/10.20517/evcna.2022.42
Chicago/Turabian Style
Ghidotti, Patrizia, Ilaria Petraroia, Orazio Fortunato, Francesca Pontis. 2023. "Immunomodulatory role of EV-derived non-coding RNA in lung cancer" Extracellular Vesicles and Circulating Nucleic Acids. 4, no.1: 59-71. http://dx.doi.org/10.20517/evcna.2022.42
ACS Style
Ghidotti, P.; Petraroia I.; Fortunato O.; Pontis F. Immunomodulatory role of EV-derived non-coding RNA in lung cancer. Extracell. Vesicles. Circ. Nucleic. Acids. 2023, 4, 59-71. http://dx.doi.org/10.20517/evcna.2022.42
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.