Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments
Abstract
Intercellular communication between diverse cell types is crucial for the maintenance of the central nervous system, and exosomes have been shown to play an important role in this process. Exosomes are small extracellular vesicles (EVs) that are released by all cell types and carry cargoes that can elicit downstream effects in recipient cells. Exosomal communication in the central nervous system has been implicated in many neurodegenerative diseases, ranging from Alzheimer’s disease to major depressive disorder. Though there remain many unknowns in the field of EV biology, in vitro experiments can provide many insights into their potential roles in health and disease. In this review, we discuss the findings of many in vitro EV experiments, with a focus on the potential roles in regulating cell viability, inflammation, oxidative stress, and neurite integrity in the central nervous system.
Keywords
INTRODUCTION
In the central nervous system (CNS), cell-to-cell communication is crucial to ensure proper development and maintenance of homeostatic conditions[1,2]. Extracellular vesicles (EVs) are membrane-bound carriers packed with proteins, metabolites, and nucleic acids (including DNA, mRNA, and microRNAs), mirroring the physiological status of the cell they originate from[3]. First observed 40 years ago[4], EVs have been shown to play a prominent role in many important cellular functions[5], giving way to an exponential increase in EV research in the past two decades. Cell-to-cell communication mediated by EVs has been implicated in many diseases ranging from several types of cancers[6] to mental illnesses such as major depressive disorder[7] and even viral infections affecting the brain and spinal cord[8].
For example, there is emerging evidence that EVs play important roles in remodeling the tumor microenvironment, including modulating macrophage activation and facilitating metabolic reprogramming of tumor-associated tissues[9]. Further, EVs have been shown to be important modulators and propagators of inflammation and neuroinflammation[10,11]. They are at least partially responsible for the spread of toxic misfolded proteins in many neurodegenerative conditions, including Alzheimer’s disease (AD) and Parkinson’s disease (PD)[12-15]. AD is the most common neurodegenerative condition, characterized clinically by memory loss, cognitive impairment, and behavioral alterations. The two major neuropathological hallmarks of AD are neurofibrillary tangles (NFTs), composed of toxic misfolded Tau protein, and amyloid beta (Aβ) plaques[16], leading to neuronal loss. PD is a neurodegenerative movement disorder characterized by the presence of α-synuclein, another toxic misfolded protein causing the degeneration of dopaminergic neurons in the substantia nigra in the midbrain[17]. Both AD and PD have been described as types of prion diseases, where misfolded proteins spread from one brain region to another, inducing further aggregation[18]. There is accumulating evidence that this spread may be mediated or exacerbated by extracellular vesicle-mediated intercellular communication[2,19-21].
Due to their ubiquity in biological processes, EVs are also being extensively studied for their therapeutic potential. Their ability to cross many membranes, including the blood-brain barrier[22], makes them appealing candidates for both non-invasive biomarker assessment and effective drug delivery devices[23]. Utilizing proteomics and machine learning, Muraoka et al. identified four EV-associated proteins (ANXA5, VGF, GPM6A, and ACTZ) that could distinguish AD patients from control patients with 88% accuracy, illustrating how powerful of a tool EVs can be in the context of biomarkers and therapeutics[24].
However, there is still much that is unknown about EV biology, and studying cell-cell communication through EVs has proven challenging given the variety of mechanisms involved[25]. For this reason, in vitro studies are crucial to understanding the biological mechanisms involved in EV formation, secretion, and uptake during normal conditions and cell-specific pathological events. Moreover, identifying molecular mechanisms involved in effecting changes in recipient cells could help identify targets for therapeutic drug development. In this review, we aim to provide an overview of current knowledge about the effects of EVs on recipient cells, with a focus on CNS-specific cell types. We first introduce general concepts on EV biogenesis, mechanisms of uptake, and current technology used to study EVs before focusing on in vitro studies investigating the effects of EV exposure on cells of the CNS in terms of cell viability, neuroinflammation, oxidative stress, and neurite integrity.
EXOSOME BIOGENESIS
All cell types, including those of the CNS, are capable of forming and secreting EVs[26-29]. Though new categories are being described, EVs can be generally subdivided, based on their size, into exosomes
Studies have suggested that small ectosomes or microvesicles that bleb directly from the plasma membrane can share many characteristics with exosomes[32]. This can make distinguishing true endosomal-origin exosomes from these small ectosomes extremely difficult, especially given that these small ectosomes seem to form at a higher rate than exosomes[32]. Since most of the studies compiled for this review likely dealt with a mixed population of EVs, we will use the terms “extracellular vesicles” and “exosomes” interchangeably when discussing the effects of “exosome” treatment on recipient cells, mostly following the language used in the cited literature.
With respect to biogenesis, true exosome formation can occur through an endosomal sorting complex required for transport (ESCRT) dependent or independent pathway[33]. In the ESCRT-dependent pathway, large, multi-subunit proteins bind to ubiquitinated proteins while causing inward budding of the endosomal membrane. Each complex in the ESCRT pathway has several ubiquitin-binding domains, which sort ubiquitinated proteins into the intraluminal vesicles (ILVs) and curate the cargo of exosomes[30]. Since ESCRT-dependent exosome formation is the most common form of exosome biogenesis[34], ESCRT-associated proteins such as ALIX and TSG101 are commonly used for exosome characterization[25].
However, ILVs are still formed when cells are depleted of ESCRT machinery[34], indicating that ESCRT-independent pathways still play major roles in exosome formation. For example, ceramide and its metabolite sphingosine-1-phosphate have been shown to be critical in sorting cargo into ILVs, independent of ESCRT machinery[33]. In this model, membrane proteins are retained into cholesterol rafts, while the increased accumulation of ceramide due to sphingomyelinase causes the inward budding of the endosomal membrane, forming ILVs without the use of ESCRT machinery[35]. Interestingly, all brain cell types have been shown to be at least partially dependent on ESCRT-independent exosomal formation[13,36,37].
In either the ESCRT-dependent or independent pathways, how the multivesicular body (MVB) is transported to the plasma membrane to secrete these exosomes is not well understood, though many Rab GTPases may play crucial roles. Ostrowski et al. conducted an RNA interference screen in HeLa cells and found that silencing Rab27a and Rab27b decreased exosome secretion[38]. Additionally, knocking down a stabilizer of Rab27a has been shown to cause a decrease in exosome secretion along with an increase in MVB size in neuronal cell lines, making these pathways relevant for the study of brain exosomes[39].
Due to their high levels of compartmentalization, polarized morphology, and excitability, exosome biogenesis and release in neurons are more nuanced than in other cells. Indeed, exosomes released from the axonal region of the neuron may carry different cargoes than exosomes released from the somatodendritic compartment[40]. Neurons are also highly excitable cells, and exosome release is regulated by synaptic activity[41], adding another layer of complexity to exosome biogenesis and secretion in neurons.
MECHANISMS OF EXOSOME UPTAKE
The two most common forms of exosome uptake into the recipient cell are clathrin-mediated endocytosis[42,43] [Figure 1A] and macropinocytosis[44] [Figure 1B]. In clathrin-mediated endocytosis, the exosomes bind to the plasma membrane and are then internalized into endosomes. Once inside the endosomal pathway, they can either be degraded in the lysosome[45] or undergo lysosomal escape and release their cargo into the cytoplasm[46]. Exosomes can also undergo back-fusion with the endosomal membrane [Figure 1F], providing another pathway for the delivery of functional cargo into the cytoplasm[42]. Macropinocytosis is a mechanism where the plasma membrane ruffles out in an actin-dependent manner and internalizes extracellular components. This has been shown to be a major form of exosome internalization in microglia, as these cells are known to perform constitutive macropinocytosis[44].
Figure 1. Extracellular vesicles can be internalized into recipient cells by different mechanisms. Image created with BioRender.
Other exosome internalization mechanisms appear to involve lipid raft-associated endocytosis [Figure 1C], as disruption of these structures by altering cholesterol dynamics can potently inhibit exosome uptake[47]. Mechanisms such as these may account for observations of exosome uptake independent of macropinocytosis and clathrin-mediated endocytosis[48]. Other forms of uptake include direct fusion with the plasma membrane [Figure 1D] and phagocytosis[14,48]. Additionally, exosomes can cause changes in recipient cells without internalization, likely through contact between integral proteins on the exosome membrane and proteins on the plasma membrane[49] [Figure 1E]. Since the cellular changes caused by exosome uptake can vary depending on which uptake mechanism is at play[14,15], understanding more about exosome uptake is crucial for the development of therapeutic strategies targeting this process.
Although all cell types can form and secrete exosomes, their uptake appears to be somewhat selective. Whether exosomes are taken up by the recipient cell can depend on a variety of factors, including donor and recipient cell types. Recent studies suggest that astrocytes cannot take up exosomes from oligodendroglia or microglia under healthy conditions[50]. Furthermore, while neuroblastoma cell lines produce exosomes that can be taken up by microglia, other neurons, and astrocytes, some studies report that primary neurons produce exosomes that are only taken up by other neurons[51], with some debate as to whether astrocytes can take them up[51,52]. Microglia, though, appear to internalize exosomes from a wide range of cell types efficiently, and they have been shown to take up exosomes from oligodendrocyte precursor cells[44], neuroblastoma cells[53], primary neurons[10], astrocytoma cells[54], primary astrocytes[55], immortalized microglia cells[56], and other primary microglia[57]. This is fitting, as microglia are commonly referred to as the sentinels of the brain[58]. Overall, the mechanisms underlying recipient cell-targeting by EVs as well as cell-dependent internalization of EVs remain somewhat elusive. It appears that the surface composition of the EVs can greatly influence the specific targeting of recipient cells. EV surface molecules such as lipids, tetraspanins, integrins, and glycoproteins have been proposed to play a role in EV tropism[30]. Studies also suggest that integrins selection and display on EV surface play a major role in EV targeting specific cell types[59].
TOOLS USED IN EXOSOME RESEARCH
Despite the increase in exosome research over the last decade, there are still many remaining challenges in the isolation, characterization, and tracking of exosomes. Various enrichment methods yielding different “purities” of exosomes have been described[60], and no one-size-fits-all method of enrichment has emerged thus far[25]. Though methods of molecular characterization of exosomes such as Western blotting, ELISA, and mass spectrometry have been used extensively, these are susceptible to contamination from other sources, and systematically identifying subpopulations based on the presence or absence of proteins remains elusive[61,62]. In terms of exosome tracking, while lipid dyes such as PKH26 have been widely used, they can form aggregates in fixed cells in the size range of exosomes, giving false positives in microscopy studies. Further, evidence suggests that cargo labeling may affect the formation and properties of the exosomes themselves, depending on the labeling method used[63].
Methods for enrichment or separation of exosomes normally rely on their size, density, or surface proteins. Therefore, they consist of size-exclusion chromatography (along with additional concentration steps), differential ultracentrifugation, and immunocapture, respectively, each with different benefits and drawbacks[64]. Innovative methods have been developed that could potentially bring better consistency and reproducibility to the exosome field. For instance, Yang et al. utilized an artificial antibody known as a “molecularly imprinted polymer” to magnetically isolate EVs, which resulted in a 3-fold enrichment of plasma exosomes compared to ultracentrifugation[65]. This method also has the potential to be customized for different capture probes with high specificity and may present a novel and precise isolation technique[66].
Tracking exosome secretion, uptake, and fate in recipient cells is also becoming more feasible with the development of innovative tagging strategies. Sung et al. recently reported a stable and more brightly fluorescent version of the molecule known as pHluorin for studying exosome formation and secretion[67]. Under acidic conditions, such as in the lysosome or MVB, pHluorin is non-fluorescent, only emitting fluorescence at neutral pH conditions, such as in the extracellular space[67]. Tagging the CD63 tetraspanin, a common marker for exosomes mainly found in EVs secreted from the endosomal pathway[32], with this new version of pHluorin, enables the visualization of exosome secretion[67]. This is important because there has been evidence showing that small ectosomes budding directly from the plasma membrane can share almost all of the same characteristics as true endosomal-origin exosomes while potentially forming in much higher numbers than true exosomes[32,62]. Tools like pHluorin that label proteins specifically based on their subcellular location can potentially help differentiate between these subpopulations of EVs.
Using the extremely bright and small bioluminescent protein nanoluciferase, O’Brien et al. were able to track the fate of internalized exosomes with high resolution, allowing them to analyze how internalized exosomes can transfer bioactive cargo to recipient cells. They found that the transfer of bioactive cargo is rare and inefficient, and their methods in tracking uptake and fate generated precise data that can be used in further studies[68].
THE EFFECTS OF EXOSOMES ON CELL VIABILITY
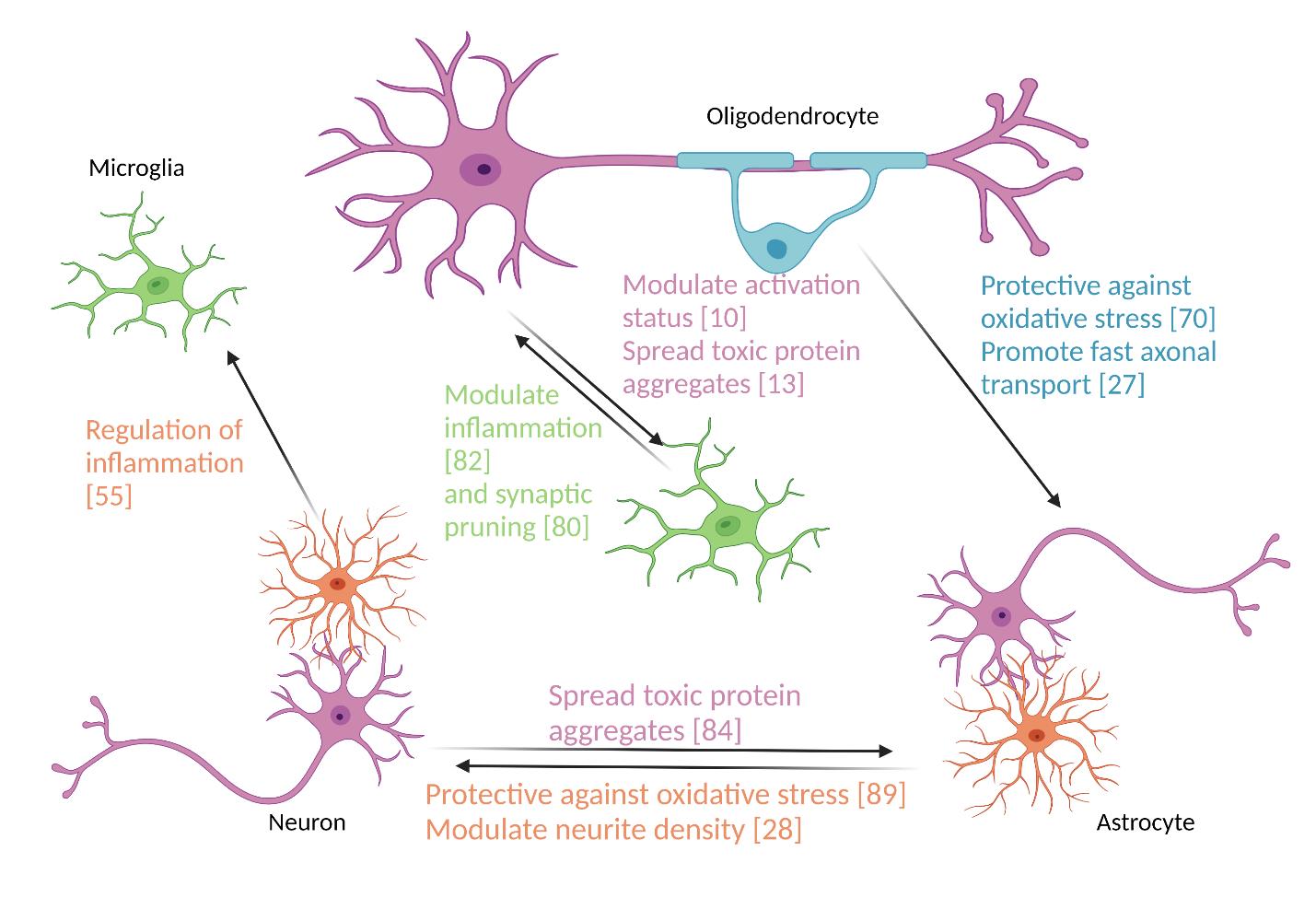
Typical measurements of cell viability include metabolic assays that use mitochondrial activity and membrane integrity as indicators of the number of living cells in a culture, as well as exclusion dye experiments that mark intracellular structures that are not enclosed in a membrane in non-viable cells as with propidium iodide which stains the DNA of non-viable cells[69]. Cell viability is one of the broadest measurements of cell health, as it simply measures the number of living cells in a population without identifying the potential mechanisms of any reduced viability. Despite these limitations, cell viability is an important starting place for studying how certain populations of exosomes elicit effects in recipient cells [Figure 2]. Here, we will focus mostly on how the cargo of glial cell exosomes affects neuronal
Figure 2. Extracellular vesicles contribute to intercellular communication in the central nervous system. Image created with BioRender.
Effects of EVs on cell viability
| Donor cells | Recipient cells | Result | Reference |
| Mouse primary oligodendrocytes | Mouse primary neurons 12-14 hrs before oxidative stress | Increased viability | [70] |
| SH-SY5Y cells with the Swe mutation of APP | CHME3 microglia | Increase in necrotic bodies initially, with a delayed increase in apoptosis | [53] |
| Total plasma EVs from AD patients | Primary rat neurons | No difference in viability | [28] |
| Astrocyte-derived plasma EVs from AD patients | Primary rat neurons | Decreased cell viability not due to apoptosis | |
| hiPSC neurons | |||
| Astrocyte-derived plasma EVs from FTLD patients | Primary rat neurons | No difference in viability | |
| hiPSC neurons | |||
| Astrocyte-derived plasma EVs from mTBI patients | PC12 | Increased cytotoxicity | [72] |
| SH-SY5Y | |||
| EVs from healthy control brain tissue | hiPSC neurons | Increased cytotoxicity | [71] |
In general, it seems as though exosomes from healthy donor glia are not inherently harmful to the survival of recipient neurons in vitro and may even elicit beneficial effects. When primary neurons were treated with exosomes from primary oligodendrocytes 12 to 14 h before undergoing an oxidative stress challenge, they exhibited substantially higher metabolic activity, indicating higher viability, when compared to treatment with exosomes from HEK293 cells, which suggests a specific function of oligodendrocyte-derived exosomes for neuronal survival of oxidative stress[70]. It is worth noting that different cell types may be more affected by exosomes than others. Indeed, exosomes enriched from the brain tissues of healthy humans caused increased cytotoxicity in mature neurons derived from hiPSCs but not in differentiated SH-SY5Y neuroblastoma cells[71]. So, while exosomes from healthy donor cells may have no effect or may even be beneficial to some recipient cells, they may also be detrimental to others, highlighting the importance of utilizing strategic controls in viability experiments with exosomes and demonstrating the complex interactions of exosomes with recipient cells.
When exosomes come from diseased donor cells, the response of recipient cells can be even more varied. In particular, astrocyte- and neuron-derived exosomes isolated from the plasma of people with mild traumatic brain injury (mTBI) caused increased cytotoxicity in recipient PC12 and SH-SY5Y neuroblastoma cells, suggesting that alterations in both neurons and glia in the brain during TBI can be propagated via exosomes[72]. Similarly, astrocyte-derived exosomes (ADEVs) from patients with AD caused decreased cell viability in recipient primary rat neurons as well as hiPSC neurons, while total serum EVs from these patients had no effect on viability, even despite the species difference. This finding was linked to high levels of complement proteins, specifically the membrane attack complex proteins, in the ADEVs and neuron-derived EVs (NDEVs), suggesting that these cargoes were crucial for the detrimental effects observed. Interestingly, this study also showed that ADEVs from patients with fronto-temporal lobular degeneration caused no decrease in cell viability in these same cultures, further pinpointing these toxic effects on AD pathology. This suggests that exosome effects can also be highly specific to the disease and donor/recipient cell types. Additionally, while there was a significant increase in necrosis in the recipient cells, there was no evidence of increased apoptosis[28]. This is consistent with a study by Fernandes et al. showing that exosomes from SH-SY5Y cells expressing the Swedish mutation (Swe) of APP (causing the cells to accumulate more
Moving forward, it will be important to couple these studies of how EVs can cause these changes in cell viability with the mechanisms involved in their uptake. Some studies have shown that, in the context of AD, the digestion of proteins on the surface of EVs can abrogate any changes in cytotoxicity caused by these EVs[28], while others have demonstrated a toxic effect of mainly luminal proteins[71]. While both factors on the exosome surface and in the exosome lumen can be considered “exosomal cargo”, it remains unknown to what extent these exosomes are decreasing viability through the delivery of luminal cargoes or through binding to receptors on the plasma membrane. Therefore, while exosomes containing specific pathologies can affect the viability of recipient cells, whether this occurs and by what mechanism it occurs depends on the cell types and disease states of both donor and recipient cells. Some of the specific pathways and mechanisms that may underlie these effects are reviewed in the next sections.
INFLAMMATION AND EXOSOMES
The inflammatory effects exosomes have on their recipient cells depend on the cell type, inflammatory status, and disease state of both the donor and recipient cells. Due to this high amount of contextual variability, it remains difficult to draw significant conclusions. Nonetheless, there are some noteworthy trends (summarized in Table 2 and Figure 2).
Effects of EVs on Inflammation
| Donor cells | Recipient cells | Result | Reference |
| Primary rat neurons | Primary rat microglia | Reduction in M1 and M2 activation | [10] |
| Protective against future LPS-stimulated activation | |||
| PC12 neurons | MG6 microglia | Upregulation of complement factor 3 | [78] |
| SH-SY5Y neurons with the Swe mutation of APP | CHME3 microglia | Increased secretion of pro-inflammatory cytokines | [53] |
| Serum-derived exosomes from patients with major depressive disorder | BV2 microglia | Increased activation | [7] |
| Microglia transfected with miR-124 mimic | Primary mouse neurons | Inhibition of inflammatory response | [80] |
| Primary astrocytes treated with brain extracts from a mouse TBI model | Primary mouse microglia | Promotion of M2 activation state | [55] |
| Primary neurons | Primary mouse microglia | Transfer of miR-124 promoting M2 activation | [81] |
| Primary mouse astrocytes | |||
| BV2 microglia in M2 activation | Primary mouse neurons | Increase of miR-124 expression | [82] |
| SH-SY5Y neurons treated with methamphetamine | U87MG astrocytes | Increased inflammatory response that is abrogated by knocking out α-synuclein | [84] |
| Brain extracts from patients with rTBI | BV2 microglia | Increased expression of miR-124, promoting M2 activation | [80] |
Microglia are important mediators of the immune response in the CNS. Microglia are constantly surveying their surroundings[73] and are vital to a diverse array of brain functions such as memory formation and synaptic pruning[74]. These roles require a constant and delicate balance between pro-inflammatory and anti-inflammatory factors[75]. This dynamic nature of microglia also extends to “activated” microglia, which respond to injury and disease through a wide spectrum of different activation states[75]. Broadly, though, there are two different states of microglial activation: the M1 and M2 states[76]. While this paradigm has been challenged in recent years[77], many studies we looked at used this terminology so that we will use the language from the literature cited as much as possible. In the M1 state, the microglia generally release pro-inflammatory cytokines and produce high amounts of reactive oxygen species, whereas, in the M2 state, the microglia generally release anti-inflammatory cytokines and engage in tissue and extracellular matrix repair[76]. Though the inflammation response in the brain is highly complex and broadly unknown, there is substantial evidence that exosomes are important in modulating microglial activity and activation.
In healthy states, neuronal exosomes may be key players in keeping microglia in a quiescent, non-inflammatory state, as exosomes from primary neurons were observed to reduce both M1 and M2 activation of recipient primary microglia and were protective against future lipopolysaccharide (LPS)-stimulated activation of the microglia[10]. In addition, PC12 neuronal cell exosomes caused an upregulation of complement factor 3 in MG6 microglia cells, which was demonstrated to be an important step in the ability of microglia to perform synaptic pruning, potentially indicating a beneficial level of inflammation[78]. These findings point to the important role of neuronal exosomes in balancing the inflammatory and resting states of microglia.
Exosomes enriched from pathology-containing tissues or cells elicit different effects on recipient cells, depending on the disease. In the case of AD, SH-SY5Y neurons engineered to express the Swe mutation of APP produced exosomes that increased the secretion of pro-inflammatory cytokines in CHME3 microglia[53]. This is consistent with other studies that have shown a correlation between Aβ levels and neuroinflammation[79]. Serum-derived exosomes from patients with major depressive disorder were also able to cause activation in recipient BV2 microglia cells[7]. Moreover, exosomes from a well-established in vitro model of depression (PC12 neurons treated with 200 mM of corticosterone), when stereotaxically injected into the hippocampus of male C57BL/6J mice, were shown to induce depression-like behaviors, as demonstrated by avoidance of the central area in an open field test. This effect was attributed to the microRNA miR-9-5p promoting microglial M1 polarization through down-regulating the protein suppressor of cytokine signaling 2 (SOCS2)[7].
In the case of TBI, BV2 microglia cells exposed to brain extracts from a repetitive traumatic brain injury (rTBI) mouse model exhibited a higher expression of microRNA miR-124-3p, which was found to promote the M2 anti-inflammatory state[80]. Jiang et al. found that neuronal exosomes could carry miR-124-3p to recipient astrocyte and microglia cells after spinal cord injury, suppressing their activated state[81]. However, other studies also demonstrated that astrocytes could promote the M2 activation state of microglia in TBI through the microRNA miR-873-5p, demonstrating that multiple cargoes could potentially be causing this effect after neurotrauma[55]. More research involving neuron-derived exosomes from in vivo models of TBI and CNS injury would be useful in identifying mechanisms affecting the activation response of microglia.
Exosomal miR-124 seems to also have a beneficial effect when communicated from microglia to neurons since exosomes from BV2 microglia in the M2 anti-inflammatory phenotype could increase the expression of miR-124 in recipient primary neurons, which was shown to be important in increasing neuronal survival under oxygen/glucose deprivation[82]. Huang et al. found that microglia transfected with a miR-124-3p mimic could inhibit the inflammatory response of primary cortical neurons by inhibiting mTOR[80]. This suggests a potential positive feedback loop, where cells packing miR-124 into exosomes can cause further expression of miR-124 in other surrounding cells.
Though astrocytes are also key regulators of immunity in the CNS, many studies report rare or no exosome uptake at all in astrocytes[44,50,51,83]. Nevertheless, it seems that under pathological conditions, they may be able to take up exosomes from some neurons. Specifically, primary neurons treated with methamphetamine produced exosomes that elicited an inflammatory response in recipient primary astrocytes, demonstrated by an increase in the production of pro-inflammatory cytokines like TNF-α, IL1β, and IL6 after exosome treatment[84]. However, this observed uptake may be due to the use of a neuroblastoma cell line, as another group found that astrocytes take up exosomes from N2A cells but not from primary neuronal cells[51]. Despite being key players in the regulation of neuroinflammation, much is unknown about whether and to what extent astrocytes modulate the inflammatory response of neurons through exosomes.
Although this review is focused on in vitro experiments, it is important to note that many similar trends are observed in vivo as well. Li et al. found that exosomes from mice stimulated with LPS, when administered intravenously to other mice, could induce microglial and astrocytic activation[85]. Our group showed that NDEV enriched from the plasma of individuals with Down syndrome elicited elevated neuroinflammation when injected into the hippocampus of wild-type mice[20]. Furthermore, exosomes from M2 microglia containing miR-124 were able to protect against neural deficits and neuronal apoptosis in a mouse model of stroke[82]. In a mouse model of spinal cord injury, NDEVs containing miR-124 were able to suppress excessive activation of microglia and astrocytes in vivo[81]. The authors linked these neuroprotective, anti-inflammatory effects of miR-124 to its ability to suppress myosin heavy chain 9, a protein known to increase inflammatory cytokine production[81].
Overall, these findings point to some trends in the complex interplay between exosomal communication and the regulation of neuroinflammation (summarized in Table 1 and Figure 1). Owing to the variability across experimental systems, it remains difficult to draw any significant conclusions, and more studies are needed to explore how exosomes communicate inflammatory responses across cell types as well as what mediates these effects. Specifically, teasing out what inflammatory responses are caused by the luminal cargo of exosomes derived from the cytoplasm of their donor cell and which responses are caused by potential surface cytokines the exosomes picked up extracellularly will be crucial.
OXIDATIVE STRESS AND EXOSOMES
The process of oxidative phosphorylation is vital for generating energy in mammalian tissues; however, as a by-product of this biochemical pathway, many reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced[86]. In brain tissue under normal conditions, these ROS and RNS molecules are important in processes such as neuronal development, long-term potentiation, and overall health, as the tissue’s antioxidant system prevents these reactive species from damaging the cell. However, under diseased conditions, this balance is disrupted, and these reactive species become toxic[86]. Because the brain is rich in peroxide-susceptible lipids and has a high energy demand, the CNS is particularly vulnerable to oxidative stress. Accordingly, oxidative stress is a feature of many neurodegenerative conditions[87]. Oxidative stress alters both the number of exosomes released as well as the cargo of these exosomes, and exosomal communication can confer both beneficial and detrimental effects on recipient cells[88], as summarized in Table 3.
Effects of EVs on oxidative stress
| Donor cells | Recipient cells | Results | Reference |
| 1321N1 human astrocytes | SH-SY5Y neurons undergoing oxidative stress | Increased viability | [90] |
| Primary astrocytes from mouse midbrain, activated with CCL3 | SH-SY5Y neurons undergoing oxidative stress | Reduction in apoptosis | [92] |
| SH-SY5Y neurons injured with MPP+ | Preservation of mitochondrial complex I | ||
| Glioblastoma cells | Primary rat neurons | Increase in oxidant status and decrease in antioxidant status | [94] |
| BV2 microglia exposed to NfL | Primary rat neurons | Increase in oxidative stress | [95] |
| Decrease in viability | |||
| Serum exosomes from a mouse model of Parkinson’s disease | Primary mouse neurons | Downregulation of OXR1 through the miRNA cargo miR-137 | [93] |
Astrocyte-derived exosomes are particularly crucial for protecting neurons against oxidative stress as they have been shown to carry prion protein, synapsin I, heat shock protein (HSP70), superoxide dismutase (SOD1), and catalase, all of which are important components of the antioxidant system in the neuron[89]. Furthermore, Pascua-Maestro et al. found that apolipoprotein D is protective against oxidative stress and is exclusively transported to neurons through exosomes[90]. This was demonstrated by the increased viability of SH-SY5Y neurons undergoing oxidative stress after being treated with the EV fractions of 1321N1 astrocytes[90].
This function of ADEVs may be vital to understanding certain neurodegenerative conditions. For example, exosomes from primary rat astrocytes decreased cognitive impairment, neuronal loss, and ROS levels when stereotaxically injected into a rat TBI model while increasing antioxidant molecules such as SOD1 and reduced glutathione[91]. Additionally, primary astrocytes isolated from the mouse ventral midbrain and striatum, when activated with the dopaminergic neuron protector molecule chemokine ligand 3 (CCL3), produced exosomes that reduced apoptosis of SH-SY5Y neurons undergoing oxidative stress[92]. This same research found that these exosomes could preserve the activity of mitochondrial complex I in SH-SY5Y cells injured with MPP+ (1-methyl-4-phenylpyridinium), a common model for studying PD, illustrating the potentially beneficial role of ADEVs in mitigating neurodegenerative diseases[92].
Exosomes can also propagate the detrimental effects of oxidative stress into other cells. For instance,
These studies illustrate the crucial role of exosome communication in both the mitigation and propagation of oxidative stress in the brain. However, there are still unanswered questions. For example, some groups have found that miR-137 can be protective against oxidative stress in astrocytes[96], directly contradicting the findings of the Jiang study, and others have found it to be key in protecting microglia against LPS inflammatory stimulation[97]. It is unclear why this particular miR can seemingly have both beneficial and detrimental effects, and studying what determines these opposite effects is crucial to deepen our understanding of exosome biology. Finally, while microglia generate high levels of ROS and contribute to oxidative stress in the brain[98], there has been comparatively little research done on how exosomes modulate microglial oxidative stress. A better understanding of how exosomes and their cargos contribute towards and fight against oxidative stress is important, considering how prevalent oxidative stress is in many neurodegenerative conditions.
NEURITE INTEGRITY
The term “neurites” refers to both axons and dendrites, and these structures are crucial to maintaining homeostasis both for individual neurons as well as for the CNS as a whole. Their formation is an actin-dependent process whereby a growth cone extends from the cell body and probes the environment around the cell for permissive elements, leading the axon to a destination where it forms a synapse[99]. These synapses then undergo selective elimination to refine neuronal connectivity in a process known as “synaptic pruning”, which occurs both during development and throughout life[78]. This is important for the appropriate wiring of neural circuits as well as for behavior, learning, and memory[78]. Once synapses are formed, the neuron can use the axon to transport proteins from the cell body out to the synapse (anterograde transport) or from the synapse back to the cell body (retrograde transport), both of which are crucial for neuronal survival[100]. Since axons can extend anywhere from a few millimeters to up to a meter away from the cell body, maintaining these structures is an energy- and resource-intensive process[100]. Failing to maintain axonal integrity is a feature in many neurodegenerative diseases such as AD, PD, and amyotrophic lateral sclerosis[101,102]. There is emerging evidence that exosomal communication in the brain plays an important role in maintaining neurite integrity [Table 4].
Exosome effects on neurite health
| Donor cells | Recipient cells | Results | Reference |
| BV2 microglia transfected with miR-124 mimic | Primary mouse neurons | Increase in neurite outgrowth | [80] |
| PC12 neurons | MG6 microglia | Increased synaptic pruning activity | [78] |
| Primary mouse oligodendrocytes | Primary mouse neurons | Promotion of fast axonal transport | [27] |
| Co-culture of primary mouse neurons, astrocytes, and oligodendrocytes exposed to Aβ fibrils | Primary mouse neurons | Severe synaptic and dendritic loss | [14] |
| Primary astrocytes stimulated with IL1β | Primary mouse neurons | Reduction in neurite length in a dose-dependent manner | [29] |
| No change in total neurites | |||
| Astrocyte-derived plasma exosomes from patients with AD | Primary rat neurons | Decrease in neurite density | [28] |
| Neuron-derived plasma exosomes from patients with AD | No effect on neurite growth | ||
| Neuron-derived plasma exosomes from TBI patients | PC12 neurons | Decrease in neurite outgrowth | [72] |
| Increase in neurite blebbing |
Beneficial effects of brain exosomes on neurite integrity include facilitating neurite outgrowth, synaptic pruning, and axonal transport. Exosomes from BV2 microglia cells expressing a mimic of miR-124-3p were able to increase neurite outgrowth of primary mouse cortical neurons[80], suggesting a beneficial effect of glia-derived exosomes on neurite outgrowth. MG6 microglia cells treated with rat PC12 neuroblastoma exosomes also performed more synaptic pruning when later co-cultured with the PC12 neurons[78], indicating that neuron-to-glia exosomal communication can also be an important mediator of synaptic pruning. Finally, exosomes from primary oligodendrocytes could promote fast axonal transport, reduce pausing time in retrograde and anterograde axonal transport, and even rescue axonal transport in neurons under starving conditions[27]. Together, these findings suggest that exosomal communication between neurons and glial cells is important in forming healthy neurites and maintaining axonal homeostasis.
In contrast, exosomes can have detrimental effects on neurite integrity in diseased states. ADEVs from patients with AD reduced neurite density in primary rat cortical neurons[28]. This was specific to astrocytes, as NDEVs from the same patients did not elicit effects on neurite outgrowth in this in vitro model[28], suggesting that differences in the cargo of ADEVs vs. NDEVs result in different outcomes in recipient cells. A co-culture of neurons, astrocytes, and oligodendrocytes exposed to Aβ fibrils for 24 h produced exosomes that caused a severe synaptic and dendritic loss in primary mouse neurons[14], suggesting that the deleterious effects of Aβ fibrils can be transferred through exosome cargo. Inflammation likely plays an important role in these effects, as You et al. found that astrocytes stimulated with IL1β caused a reduction in neurite length of primary murine neurons in a dose-dependent manner[29]. Interestingly, the authors did not find that the exosomes decreased the total number of neurites in vitro[29], which suggests that these exosomes could be specifically targeting pathways involved in neurite outgrowth. These data show compelling evidence for the role of ADEVs in synaptic and dendritic dysfunction in AD.
Though glial exosomes, particularly from astrocytes, are clearly important modulators in neurite integrity and function, NDEVs from patients with TBI were shown to cause a decrease in neurite outgrowth and an increase in neurite blebbing in a neuroblastoma cell line[103], highlighting the potential role of NDEVs in neurite health.
Replicating these findings in vivo, Asai et al. found that depleting mouse brains of microglia resulted in a suppression of Tau spreading, linking axonal health to inflammatory mediators of the CNS. The authors further presented evidence that these microglia were able to spread Tau specifically through exosomes[13], demonstrating how exosomes can spread neurite-damaging proteins between cells. Another group showed that primary mouse neurons produced exosomes that increased the proliferation and differentiation of neurons in mice in vivo, further highlighting the importance of exosomes in neuronal development[104]. These pieces of evidence show that information gained from in vitro studies of how exosomes modulate neurite integrity can extend to in vivo models.
Maintaining functional neurites is crucial to neuronal survival as well as to maintaining homeostasis. Though neurite formation and stability are affected by complex mechanisms, exosomal communication is a major mechanism by which these structures are modulated.
CONCLUSION AND FUTURE DIRECTIONS
This review highlights the major progress made toward a better understanding of EV function in the CNS using in vitro models. While exosome research has seen a spectacular increase over the last decade, some challenges remain. More studies are needed to improve the knowledge of the different EV CNS subpopulations in terms of biogenesis and secretion, as well as uptake mechanisms, but also in terms of size, cargo composition, and specific markers. Since EVs play a significant dual role in cell-cell communication in neurodegenerative diseases, it is important to gain better knowledge about their potential beneficial and detrimental effects in different disease conditions. This could significantly contribute to the development of specific therapeutic avenues targeting, for example, the spread of inflammation and misfolded proteins that are characteristic of many neurodegenerative conditions.
There are many new research directions that can increase reproducibility in experimental systems as well as broaden our knowledge of EV biology. Co-cultures of neurons and glia, along with 3D organoid systems, can help elucidate how EV communication modulates different cell types in a more physiologically relevant model, potentially decreasing experiment-to-experiment variability. Utilizing organoids will be particularly interesting, as cells grown in 3D have been shown to have different rates of exosome secretion as well as different exosomal cargos[105], and seem to produce EVs that are more closely related to EV produced in in vivo tissue systems than 2D cultures[106]. 3D organoids can also model different stages of organ development, which could potentially reveal differential EV characteristics in different stages of growth[107,108].
Finally, there are many big questions in exosome research that remain unanswered. For example, does the endosomal origin of “true exosomes” cause them to have different effects when compared to small ectosomes or other non-endosomal extracellular vesicles?[62] Could true endosomal exosomes have different effects on cell viability, inflammation, oxidative stress, and neurite health when compared to microvesicles? As a robust method has yet to be developed to ascertain the endosomal origin of EVs, this currently remains unknown. Moreover, in studying how exosomes modulate the inflammatory response of recipient cells, it will be important to determine whether exosomes carry cytokines in their cargo or whether the cytokines are attached to the outer surface of the membrane[109].
DECLARATIONS
AcknowledgmentsThe authors wish to thank Dr. Lotta Granholm for her insightful comments on the manuscript.
Author’s contributionsConceptualization, writing-original draf, and investigation: Colvett I, Ledreux A
Review and editing: Saternos H, Coughlan C, Vielle A, Ledreux A
Visualization: Colvett I
Availability of data and materialsNot Applicable.
Financial support and sponsorshipThis work was made possible by support from the NIA (grants R01 AG070153 and R01 AG071228 to AL).
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateNot Applicable.
Consent for publicationNot Applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci 2014;369:20130516.
2. Sharma P, Schiapparelli L, Cline HT. Exosomes function in cell-cell communication during brain circuit development. Curr Opin Neurobiol 2013;23:997-1004.
3. Frühbeis C, Fröhlich D, Krämer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol 2012;3:119.
4. Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol 2013;200:367-71.
5. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020:367.
6. Tai YL, Chen KC, Hsieh JT, Shen TL. Exosomes in cancer development and clinical applications. Cancer Sci 2018;109:2364-74.
7. Xian X, Cai LL, Li Y, et al. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology 2022;20:122.
8. Sampey GC, Meyering SS, Zadeh MA, Saifuddin M, Hakami RM, Kashanchi F. Exosomes and their role in CNS viral infections. J Neurovirol 2014;20:199-208.
9. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther 2020;5:242.
10. Peng H, Harvey BT, Richards CI, Nixon K. Neuron-derived extracellular vesicles modulate microglia activation and function. Biology (Basel) 2021;10:948.
12. Alvarez-Erviti L, Seow Y, Schapira AH, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 2011;42:360-7.
13. Asai H, Ikezu S, Tsunoda S, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 2015;18:1584-93.
14. Beretta C, Nikitidou E, Streubel-Gallasch L, Ingelsson M, Sehlin D, Erlandsson A. Extracellular vesicles from amyloid-β exposed cell cultures induce severe dysfunction in cortical neurons. Sci Rep 2020;10:19656.
15. Wang Y, Balaji V, Kaniyappan S, et al. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 2017;12:5.
17. Armstrong MJ, Okun MS. Diagnosis and treatment of parkinson disease: a review. JAMA 2020;323:548-60.
18. Ayers JI, Paras NA, Prusiner SB. Expanding spectrum of prion diseases. Emerg Top Life Sci 2020;4:155-67.
19. Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J Biol Chem 2016;291:12445-66.
20. Ledreux A, Thomas S, Hamlett ED, et al. Small neuron-derived extracellular vesicles from individuals with down syndrome propagate Tau pathology in the wildtype mouse brain. J Clin Med 2021;10:3931.
21. Ruan Z, Pathak D, Venkatesan Kalavai S, et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 2021;144:288-309.
22. Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 2021;13:122.
23. Mathew B, Mansuri MS, Williams KR, Nairn AC. Exosomes as emerging biomarker tools in neurodegenerative and neuropsychiatric disorders-a proteomics perspective. Brain Sci 2021;11:258.
24. Muraoka S, DeLeo AM, Sethi MK, et al. Proteomic and biological profiling of extracellular vesicles from Alzheimer’s disease human brain tissues. Alzheimers Dement 2020;16:896-907.
25. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750.
26. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8:727.
27. Frühbeis C, Kuo-Elsner WP, Müller C, et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol 2020;18:e3000621.
28. Nogueras-Ortiz CJ, Mahairaki V, Delgado-Peraza F, et al. Astrocyte- and neuron-derived extracellular vesicles from Alzheimer’s disease patients effect complement-mediated neurotoxicity. Cells 2020;9:1618.
29. You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles 2020;9:1706801.
30. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021;19:47.
31. Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 2015;4:27066.
32. Fordjour FK, Guo C, Ai Y, Daaboul GG, Gould SJ. A shared, stochastic pathway mediates exosome protein budding along plasma and endosome membranes. J Biol Chem 2022;298:102394.
33. Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun 2013;4:2712.
34. Wei D, Zhan W, Gao Y, et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res 2021;31:157-77.
35. Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 2015;290:3455-67.
36. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008;319:1244-7.
37. Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J Biol Chem 2012;287:10977-89.
38. Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19-30; sup pp 1.
39. Song L, Tang S, Han X, et al. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun 2019;10:1639.
40. Blanchette CR, Rodal AA. Mechanisms for biogenesis and release of neuronal extracellular vesicles. Curr Opin Neurobiol 2020;63:104-10.
41. Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 2006;31:642-8.
42. Joshi BS, de Beer MA, Giepmans BNG, Zuhorn IS. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 2020;14:4444-55.
43. Laulagnier K, Javalet C, Hemming FJ, et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci 2018;75:757-73.
44. Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 2011;124:447-58.
45. Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem 2014;289:22258-67.
46. Polanco JC, Hand GR, Briner A, Li C, Götz J. Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol 2021;141:235-56.
47. Plebanek MP, Mutharasan RK, Volpert O, Matov A, Gatlin JC, Thaxton CS. nanoparticle targeting and cholesterol flux through scavenger receptor type B-1 inhibits cellular exosome uptake. Sci Rep 2015;5:15724.
48. Delenclos M, Trendafilova T, Mahesh D, et al. Investigation of endocytic pathways for the internalization of exosome-associated oligomeric alpha-synuclein. Front Neurosci 2017;11:172.
49. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 2012;31:1231-40.
50. Ogaki A, Ikegaya Y, Koyama R. Extracellular vesicles taken up by astrocytes. Int J Mol Sci 2021;22:10553.
51. Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 2014;3:24722.
52. Morel L, Regan M, Higashimori H, et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem 2013;288:7105-16.
53. Fernandes A, Ribeiro AR, Monteiro M, Garcia G, Vaz AR, Brites D. Secretome from SH-SY5Y APP(Swe) cells trigger time-dependent CHME3 microglia activation phenotypes, ultimately leading to miR-21 exosome shuttling. Biochimie 2018;155:67-82.
54. Yang L, Niu F, Yao H, et al. Exosomal miR-9 released from HIV Tat stimulated astrocytes mediates microglial migration. J Neuroimmune Pharmacol 2018;13:330-44.
55. Long X, Yao X, Jiang Q, et al. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation 2020;17:89.
56. Grimaldi A, Serpe C, Chece G, et al. Microglia-derived microvesicles affect microglia phenotype in glioma. Front Cell Neurosci 2019;13:41.
57. Verderio C, Muzio L, Turola E, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 2012;72:610-24.
58. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol 2014;32:367-402.
59. Edelmann MJ, Kima PE. Current understanding of extracellular vesicle homing/tropism. Zoonoses (Burlingt) 2022;2:14.
60. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci 2020;21:6466.
61. Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng 2019;3:011503.
62. Mathieu M, Névo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 2021;12:4389.
63. Verweij FJ, Balaj L, Boulanger CM, et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods 2021;18:1013-26.
64. Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 2020;10:1039.
65. Yang J, Pan B, Zeng F, et al. Magnetic colloid antibodies accelerate small extracellular vesicles isolation for point-of-care diagnostics. Nano Lett 2021;21:2001-9.
66. Xu K, Jin Y, Li Y, Huang Y, Zhao R. Recent progress of exosome isolation and peptide recognition-guided strategies for exosome research. Front Chem 2022;10:844124.
67. Sung BH, von Lersner A, Guerrero J, et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun 2020;11:2092.
68. O'Brien K, Ughetto S, Mahjoum S, Nair AV, Breakefield XO. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep 2022;39:110651.
69. Cummings BS, Schnellmann RG. Measurement of cell death in mammalian cells. Curr Protoc Pharmacol 2004;Chapter 12:Unit 12.8.
70. Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 2013;11:e1001604.
71. Sardar Sinha M, Ansell-Schultz A, Civitelli L, et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol 2018;136:41-56.
72. Winston CN, Romero HK, Ellisman M, et al. Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci 2019;13:1005.
73. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314-8.
74. Nguyen PT, Dorman LC, Pan S, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 2020;182:388-403.e15.
75. Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation 2021;18:258.
76. Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 2016;53:1181-94.
78. Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep 2015;5:7989.
79. Ismail R, Parbo P, Madsen LS, et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J Neuroinflammation 2020;17:151.
80. Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 2018;32:512-28.
81. Jiang D, Gong F, Ge X, et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology 2020;18:105.
82. Song Y, Li Z, He T, et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019;9:2910-23.
83. Venturini A, Passalacqua M, Pelassa S, et al. Exosomes from astrocyte processes: signaling to neurons. Front Pharmacol 2019;10:1452.
84. Meng Y, Ding J, Li C, Fan H, He Y, Qiu P. Transfer of pathological α-synuclein from neurons to astrocytes via exosomes causes inflammatory responses after METH exposure. Toxicol Lett 2020;331:188-99.
85. Li JJ, Wang B, Kodali MC, et al.
87. Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep 2016;4:519-22.
88. Chiaradia E, Tancini B, Emiliani C, et al. Extracellular vesicles under oxidative stress conditions: biological properties and physiological roles. Cells 2021;10:1763.
89. Ranjit S, Patters BJ, Gerth KA, Haque S, Choudhary S, Kumar S. Potential neuroprotective role of astroglial exosomes against smoking-induced oxidative stress and HIV-1 replication in the central nervous system. Expert Opin Ther Targets 2018;22:703-14.
90. Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D. Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front Cell Neurosci 2018;12:526.
91. Zhang W, Hong J, Zhang H, Zheng W, Yang Y. Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis. Aging (Albany NY) 2021;13:21642-58.
92. Leggio L, L'Episcopo F, Magrì A, et al. Small extracellular vesicles secreted by nigrostriatal astrocytes rescue cell death and preserve mitochondrial function in Parkinson’s disease. Adv Healthc Mater 2022;11:e2201203.
93. Jiang Y, Liu J, Chen L, et al. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res 2019;1722:146331.
94. Genc S, Pennisi M, Yeni Y, et al. Potential neurotoxic effects of glioblastoma-derived exosomes in primary cultures of cerebellar neurons via oxidant stress and glutathione depletion. Antioxidants (Basel) 2022;11:1225.
95. Gong L, Yu Q, Wang H, et al. Neurofilament light chain (NF-L) stimulates lipid peroxidation to neuronal membrane through microglia-derived ferritin heavy chain [FTH) secretion. Oxid Med Cell Longev 2022;2022:3938940.
96. Tian R, Wu B, Fu C, Guo K. miR-137 prevents inflammatory response, oxidative stress, neuronal injury and cognitive impairment via blockade of Src-mediated MAPK signaling pathway in ischemic stroke. Aging (Albany NY) 2020;12:10873-95.
97. Gao F, Lei J, Zhang Z, Yang Y, You H. Curcumin alleviates LPS-induced inflammation and oxidative stress in mouse microglial BV2 cells by targeting miR-137-3p/NeuroD1. RSC Adv 2019;9:38397-406.
98. Simpson DSA, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel) 2020;9:743.
99. Miller KE, Suter DM. An integrated cytoskeletal model of neurite outgrowth. Front Cell Neurosci 2018;12:447.
100. Muzio MR, Cascella M. Histology, axon. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
101. Krauss R, Bosanac T, Devraj R, Engber T, Hughes RO. Axons matter: the promise of treating neurodegenerative disorders by targeting SARM1-mediated axonal degeneration. Trends Pharmacol Sci 2020;41:281-93.
102. Salvadores N, Gerónimo-Olvera C, Court FA. Axonal Degeneration in AD: the contribution of Aβ and Tau. Front Aging Neurosci 2020;12:581767.
103. Winston CN, Aulston B, Rockenstein EM, et al. Neuronal exosome-derived human tau is toxic to recipient mouse neurons
104. Sharma P, Mesci P, Carromeu C, et al. Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA 2019;116:16086-94.
105. Bordanaba-Florit G, Madarieta I, Olalde B, Falcón-Pérez JM, Royo F. 3D cell cultures as prospective models to study extracellular vesicles in cancer. Cancers (Basel) 2021;13:307.
106. Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of
107. Cui K, Chen W, Cao R, et al. Brain organoid-on-chip system to study the effects of breast cancer derived exosomes on the neurodevelopment of brain. Cell Regen 2022;11:7.
108. Zhou J, Flores-Bellver M, Pan J, et al. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci Rep 2021;11:21128.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Colvett I, Saternos H, Coughlan C, Vielle A, Ledreux A. Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments. Extracell Vesicles Circ Nucleic Acids 2023;4:72-89. http://dx.doi.org/10.20517/evcna.2023.07
AMA Style
Colvett I, Saternos H, Coughlan C, Vielle A, Ledreux A. Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments. Extracellular Vesicles and Circulating Nucleic Acids. 2023; 4(1): 72-89. http://dx.doi.org/10.20517/evcna.2023.07
Chicago/Turabian Style
Colvett, Isaac, Hannah Saternos, Christina Coughlan, Anne Vielle, Aurélie Ledreux. 2023. "Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments" Extracellular Vesicles and Circulating Nucleic Acids. 4, no.1: 72-89. http://dx.doi.org/10.20517/evcna.2023.07
ACS Style
Colvett, I.; Saternos H.; Coughlan C.; Vielle A.; Ledreux A. Extracellular vesicles from the CNS play pivotal roles in neuroprotection and neurodegeneration: lessons from in vitro experiments. Extracell. Vesicles. Circ. Nucleic. Acids. 2023, 4, 72-89. http://dx.doi.org/10.20517/evcna.2023.07
About This Article
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.